Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver
- PMID: 21294146
- PMCID: PMC3078645
- DOI: 10.1002/hep.24119
Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver
Abstract
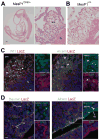
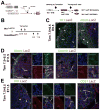
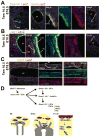
The septum transversum mesenchyme (STM) signals to induce hepatogenesis from the foregut endoderm. Hepatic stellate cells (HSCs) are sinusoidal pericytes assumed to originate from the STM and participate in mesenchymal-epithelial interaction in embryonic and adult livers. However, the developmental origin of HSCs remains elusive due to the lack of markers for STM and HSCs. We previously identified submesothelial cells (SubMCs) beneath mesothelial cells (MCs) as a potential precursor for HSCs in developing livers. In the present study, we reveal that both STM in embryonic day (E) 9.5 and MC/SubMCs in E12.5 share the expression of activated leukocyte cell adhesion molecule (Alcam), desmin, and Wilms tumor 1 homolog (Wt1). A cell lineage analysis using MesP1(Cre) /Rosa26lacZ(flox) mice identifies the mesodermal origin of the STM, HSCs, and perivascular mesenchymal cells (PMCs). A conditional cell lineage analysis using the Wt1(CreERT2) mice demonstrates that Wt1(+) STM gives rise to MCs, SubMCs, HSCs, and PMCs during liver development. Furthermore, we find that Wt1(+) MC/SubMCs migrate inward from the liver surface to generate HSCs and PMCs including portal fibroblasts, smooth muscle cells, and fibroblasts around the central veins. On the other hand, the Wt1(+) STM and MC/SubMCs do not contribute to sinusoidal endothelial cells, Kupffer cells, and hepatoblasts.
Conclusion: our results demonstrate that HSCs and PMCs are derived from MC/SubMCs, which are traced back to mesodermal STM during liver development.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures




References
-
- Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. - PubMed
-
- Bhunchet E, Wake K. Role of mesenchymal cell populations in porcine serum-induced rat liver fibrosis. Hepatology. 1992;16:1452–1473. - PubMed
-
- Zarets KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002;3:499–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
