Development and applications of fluorogenic probes for mercury(II) based on vinyl ether oxymercuration
- PMID: 21294513
- PMCID: PMC3045475
- DOI: 10.1021/ja108028m
Development and applications of fluorogenic probes for mercury(II) based on vinyl ether oxymercuration
Abstract
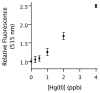
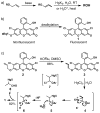
Mercury is a major threat to the environment and to human health. It is highly desirable to develop a user-friendly kit for on-site mercury detection. Such a method must be able to detect mercury below the threshold levels for drinking water, 1-2 ppb. We developed a fluorescence method based on the oxymercuration of vinyl ethers to detect mercury in dental and environmental samples. Chloride ions interfered with the oxymercuration reaction, but the addition of AgNO(3) solved this problem. Fine electronic and structural tuning led to the development of a more responsive probe that was less sensitive to chloride ion interference. This second-generation probe could detect 1 ppb mercury ions in water.
Figures




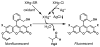
Similar articles
-
A highly selective and sensitive fluorescent probe for quantitative detection of Hg(2+) based on aggregation-induced emission features.Talanta. 2015 Jan;132:864-70. doi: 10.1016/j.talanta.2014.10.048. Epub 2014 Oct 31. Talanta. 2015. PMID: 25476389
-
A cryptand based chemodosimetric probe for naked-eye detection of mercury(II) ion in aqueous medium and its application in live cell imaging.Chem Commun (Camb). 2009 Aug 7;(29):4417-9. doi: 10.1039/b907646h. Epub 2009 Jun 12. Chem Commun (Camb). 2009. PMID: 19597611
-
A carbonothioate-based highly selective fluorescent probe with a large Stokes shift for detection of Hg2.Luminescence. 2018 Feb;33(1):219-224. doi: 10.1002/bio.3404. Epub 2017 Oct 25. Luminescence. 2018. PMID: 29068523
-
Vinyl substituted triphenylamine based turn-off fluorescent probe for selective and sensitive detection of mercury (II) in water and live cells.Spectrochim Acta A Mol Biomol Spectrosc. 2023 Jan 15;285:121887. doi: 10.1016/j.saa.2022.121887. Epub 2022 Sep 17. Spectrochim Acta A Mol Biomol Spectrosc. 2023. PMID: 36162211
-
Recent Progress in Small-Molecule Fluorescent Probes for Detecting Mercury Ions.Crit Rev Anal Chem. 2022;52(2):250-274. doi: 10.1080/10408347.2020.1797466. Epub 2020 Jul 26. Crit Rev Anal Chem. 2022. PMID: 32715731 Review.
Cited by
-
4-Mercaptopyridine-Modified Sensor for the Sensitive Electrochemical Detection of Mercury Ions.Micromachines (Basel). 2023 Mar 27;14(4):739. doi: 10.3390/mi14040739. Micromachines (Basel). 2023. PMID: 37420972 Free PMC article.
-
Synthesis of pyridine-based 1,3,4-oxadiazole derivative as fluorescence turn-on sensor for high selectivity of Ag+.J Fluoresc. 2013 Jul;23(4):785-91. doi: 10.1007/s10895-013-1213-y. Epub 2013 Mar 16. J Fluoresc. 2013. PMID: 23504218
-
A Fast-Responsive Fluorescent Sensor for Hg(2+) with High Selectivity and Sensitivity in Aqueous Media.J Fluoresc. 2015 Nov;25(6):1543-8. doi: 10.1007/s10895-015-1670-6. Epub 2015 Sep 23. J Fluoresc. 2015. PMID: 26399540
-
Fluorescent Chemosensors for Various Analytes Including Reactive Oxygen Species, Biothiol, Metal Ions, and Toxic Gases.ACS Omega. 2018 Oct 19;3(10):13731-13751. doi: 10.1021/acsomega.8b01717. eCollection 2018 Oct 31. ACS Omega. 2018. PMID: 31458074 Free PMC article. Review.
-
Target-triggered cascade assembly of a catalytic network as an artificial enzyme for highly efficient sensing.Chem Sci. 2017 Jul 1;8(7):4833-4839. doi: 10.1039/c7sc01453h. Epub 2017 Apr 28. Chem Sci. 2017. PMID: 28959405 Free PMC article.
References
-
- Atchison WD, Hare MF. FASEB J. 1994;8:622–629. - PubMed
-
- Nierenberg DW, Nordgren RE, Chang MB, Siegler RW, Blayney MB, Hochberg F, Toribara TY, Cernichiari E, Clarkson T. N Engl J Med. 1998;338:1672–1676. - PubMed
-
-
To comply with a moratorium at our institution, this work does not involve the deliberate use of organic mercury compounds.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

