Direct observation and quantitative characterization of singlet oxygen in aqueous solution upon UVA excitation of 6-thioguanines
- PMID: 21294562
- PMCID: PMC3079460
- DOI: 10.1021/jp109590t
Direct observation and quantitative characterization of singlet oxygen in aqueous solution upon UVA excitation of 6-thioguanines
Abstract
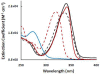
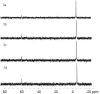
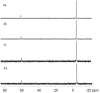
The incorporation of 6-thioguanine (6-TG) into DNA increases the risk of (1)O(2)-initiated skin cancer. We herein provide the first report on quantitative characterization of the photoactivity of 6-thioguanines including 6-TG and 6-thioguanosine. Time-resolved singlet oxygen luminescence was observed directly for the first time after UVA irradiation of 6-thioguanines in both CHCN(3) and aqueous solutions. Their photosensitization was characterized by the quantum yield of singlet oxygen production, showing a dramatic decrease over time from the initial 0.49-0.58 to zero. Experiments performed on both 6-TG and 6-thioguanosine did not show any significant difference in the quantum yield of singlet oxygen production, indicating that there was no potential participation of 7H- and 9H-tautomers. Our findings provide a primary basis for a better understanding of molecular events of thiopurine drugs in biological systems.
© 2011 American Chemical Society
Figures

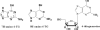
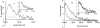
Similar articles
-
Absorption Characteristics and Quantum Yields of Singlet Oxygen Generation of Thioguanosine Derivatives.Photochem Photobiol. 2018 Jul;94(4):677-684. doi: 10.1111/php.12900. Epub 2018 Mar 31. Photochem Photobiol. 2018. PMID: 29420844
-
Formation of guanine-6-sulfonate from 6-thioguanine and singlet oxygen: a combined theoretical and experimental study.J Am Chem Soc. 2013 Mar 20;135(11):4509-15. doi: 10.1021/ja400483j. Epub 2013 Mar 8. J Am Chem Soc. 2013. PMID: 23444959
-
UVA and endogenous photosensitizers--the detection of singlet oxygen by its luminescence.Photochem Photobiol Sci. 2012 Jan;11(1):107-17. doi: 10.1039/c1pp05142c. Epub 2011 Oct 11. Photochem Photobiol Sci. 2012. PMID: 21986813 Review.
-
Excited-State Dynamics of the Thiopurine Prodrug 6-Thioguanine: Can N9-Glycosylation Affect Its Phototoxic Activity?Molecules. 2017 Feb 28;22(3):379. doi: 10.3390/molecules22030379. Molecules. 2017. PMID: 28264514 Free PMC article.
-
Multiple forms of DNA damage caused by UVA photoactivation of DNA 6-thioguanine.Photochem Photobiol. 2012 Jan-Feb;88(1):5-13. doi: 10.1111/j.1751-1097.2011.01043.x. Epub 2011 Dec 16. Photochem Photobiol. 2012. PMID: 22077233 Review.
Cited by
-
Quantification of Thiopurine/UVA-Induced Singlet Oxygen Production.J Photochem Photobiol A Chem. 2011 Oct 15;224(1):16-24. doi: 10.1016/j.jphotochem.2011.09.001. J Photochem Photobiol A Chem. 2011. PMID: 22081749 Free PMC article.
-
Identification of an RNA-binding perturbing characteristic for thiopurine drugs and their derivatives to disrupt CELF1-RNA interaction.Nucleic Acids Res. 2024 Oct 14;52(18):10810-10822. doi: 10.1093/nar/gkae788. Nucleic Acids Res. 2024. PMID: 39268573 Free PMC article.
-
Wavelength dependence of ultraviolet radiation-induced DNA damage as determined by laser irradiation suggests that cyclobutane pyrimidine dimers are the principal DNA lesions produced by terrestrial sunlight.FASEB J. 2011 Sep;25(9):3079-91. doi: 10.1096/fj.11-187336. Epub 2011 May 25. FASEB J. 2011. PMID: 21613571 Free PMC article.
-
Synthesis and photophysics of an octathioglycosylated zinc(II) phthalocyanine.Tetrahedron Lett. 2011 Oct 19;52(42):5456-5459. doi: 10.1016/j.tetlet.2011.08.028. Tetrahedron Lett. 2011. PMID: 21966031 Free PMC article.
-
MS-CASPT2 Studies on the Photophysics of Selenium-Substituted Guanine Nucleobase.ACS Omega. 2019 Jun 4;4(6):9769-9777. doi: 10.1021/acsomega.9b01276. eCollection 2019 Jun 30. ACS Omega. 2019. PMID: 31460068 Free PMC article.
References
-
- Aarbakke Jarle, Janka-Schaub Gritta, Elion GB. Trends in Pharmacological Sciences. 1997;18:3–8. - PubMed
-
- Relling Mary V., Dervieux T. Nature Reviews Cancer. 2001;1:99–108. - PubMed
-
- Middleton Chris T., de La Harpe Kimberly, Su Charlene, Law Yu Kay, Crespo-Hernández Carlos E., Kohler B. Annual Review of Physical Chemistry. 2009;60:217–239. - PubMed
-
- Crespo-Hernández Carlos E., Cohen Boiko, Hare Patrick M., Kohler a. B. Chem. Rev. 2004;104:1977–2020. - PubMed
-
- Milder Steven J., Kliger DS. J. Am. Chem. Soc. 1985;107:7365–7373.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

