Lipid raft redox signaling: molecular mechanisms in health and disease
- PMID: 21294649
- PMCID: PMC3135227
- DOI: 10.1089/ars.2010.3619
Lipid raft redox signaling: molecular mechanisms in health and disease
Abstract
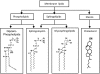
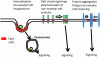
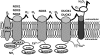
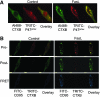
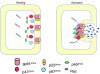
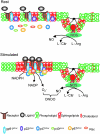
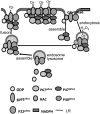
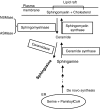
Lipid rafts, the sphingolipid and cholesterol-enriched membrane microdomains, are able to form different membrane macrodomains or platforms upon stimulations, including redox signaling platforms, which serve as a critical signaling mechanism to mediate or regulate cellular activities or functions. In particular, this raft platform formation provides an important driving force for the assembling of NADPH oxidase subunits and the recruitment of other related receptors, effectors, and regulatory components, resulting, in turn, in the activation of NADPH oxidase and downstream redox regulation of cell functions. This comprehensive review attempts to summarize all basic and advanced information about the formation, regulation, and functions of lipid raft redox signaling platforms as well as their physiological and pathophysiological relevance. Several molecular mechanisms involving the formation of lipid raft redox signaling platforms and the related therapeutic strategies targeting them are discussed. It is hoped that all information and thoughts included in this review could provide more comprehensive insights into the understanding of lipid raft redox signaling, in particular, of their molecular mechanisms, spatial-temporal regulations, and physiological, pathophysiological relevances to human health and diseases.
Figures



References
-
- Abdel Shakor AB. Kwiatkowska K. Sobota A. Cell surface ceramide generation precedes and controls FcgammaRII clustering and phosphorylation in rafts. J Biol Chem. 2004;279:36778–36787. - PubMed
-
- Abrahamsen H. Baillie G. Ngai J. Vang T. Nika K. Ruppelt A. Mustelin T. Zaccolo M. Houslay M. Tasken K. TCR- and CD28-mediated recruitment of phosphodiesterase 4 to lipid rafts potentiates TCR signaling. J Immunol. 2004;173:4847–4858. - PubMed
-
- Adam D. Heinrich M. Kabelitz D. Schutze S. Ceramide: does it matter for T cells? Trends Immunol. 2002;23:1–4. - PubMed
-
- Ago T. Kitazono T. Kuroda J. Kumai Y. Kamouchi M. Ooboshi H. Wakisaka M. Kawahara T. Rokutan K. Ibayashi S. Iida M. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

