Vaccination with human anti-trastuzumab anti-idiotype scFv reverses HER2 immunological tolerance and induces tumor immunity in MMTV.f.huHER2(Fo5) mice
- PMID: 21294885
- PMCID: PMC3109586
- DOI: 10.1186/bcr2826
Vaccination with human anti-trastuzumab anti-idiotype scFv reverses HER2 immunological tolerance and induces tumor immunity in MMTV.f.huHER2(Fo5) mice
Abstract
Introduction: Novel adjuvant therapies are needed to prevent metastatic relapses in HER2-expressing breast cancer. Here, we tested whether trastuzumab-selected single-chain Fv (scFv) could be used to develop an anti-idiotype-based vaccine to inhibit growth of HER2-positive tumor cells in vitro and in vivo through induction of long-lasting HER-specific immunity.
Methods: BALB/c mice were immunized with anti-trastuzumab anti-idiotype (anti-Id) scFv (scFv40 and scFv69), which mimic human HER2. Their sera were assessed for the presence of HER2-specific Ab1' antibodies and for their ability to reduce viability of SK-OV-3 cells, a HER2-positive cancer cell line, in nude mice. MMTV.f.huHER2(Fo5) transgenic mice were immunized with scFv40 and scFv69 and, then, growth inhibition of spontaneous HER2-positive mammary tumors, humoral response, antibody isotype as well as splenocyte secretion of IL2 and IFN-γ were evaluated.
Results: Adoptively-transferred sera from BALB/c mice immunized with scFv40 and scFv69 contain anti-HER2 Ab1' antibodies that can efficiently inhibit growth of SK-OV-3 cell tumors in nude mice. Similarly, prophylactic vaccination with anti-Id scFv69 fully protects virgin or primiparous FVB-MMTV.f.huHER2(Fo5) females from developing spontaneous mammary tumors. Moreover, such vaccination elicits an anti-HER2 Ab1' immune response together with a scFv69-specific Th1 response with IL2 and IFN-γ cytokine secretion.
Conclusions: Anti-trastuzumab anti-Id scFv69, used as a therapeutic or prophylactic vaccine, protects mice from developing HER2-positive mammary tumors by inducing both anti-HER2 Ab1' antibody production and an anti-HER2 Th2-dependent immune response. These results suggest that scFv69 could be used as an anti-Id-based vaccine for adjuvant therapy of patients with HER2-positive tumors to reverse immunological tolerance to HER2.
Figures
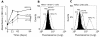
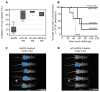



References
-
- Martin M, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, Munarriz B, Rodriguez CA, Crespo C, de Alava E, Lopez Garcia-Asenjo JA, Guitian MD, Almenar S, Gonzalez-Palacios JF, Vera F, Palacios J, Ramos M, Garcia Marco JM, Lluch A, Alvarez I, Segui MA, Mayordomo JI, Anton A, Baena JM, Plazaola A, Modolell A, Pelegri A, Mel JR, Aranda E, Adrover E. et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J Natl Cancer Inst. 2008;100:805–814. doi: 10.1093/jnci/djn151. - DOI - PubMed
-
- Disis ML, Schiffman K, Salazar LG, Almand B, Knutson KL. HER-2/neu vaccines. Cancer Chemother Biol Response Modif. 2003;21:275–285. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

