Identification of alternatively spliced Dab1 and Fyn isoforms in pig
- PMID: 21294906
- PMCID: PMC3044655
- DOI: 10.1186/1471-2202-12-17
Identification of alternatively spliced Dab1 and Fyn isoforms in pig
Abstract
Background: Disabled-1 (Dab1) is an adaptor protein that is essential for the intracellular transduction of Reelin signaling, which regulates the migration and differentiation of postmitotic neurons during brain development in vertebrates. Dab1 function depends on its tyrosine phosphorylation by Src family kinases, especially Fyn.
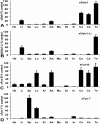
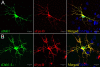
Results: We have isolated alternatively spliced forms of porcine Dab1 from brain (sDab1) and liver (sDab1-Li) and Fyn from brain (sFyn-B) and spleen (sFyn-T). Radiation hybrid mapping localized porcine Dab1 (sDab1) and Fyn (sFyn) to chromosomes 6q31-35 and 1p13, respectively. Real-time quantitative RT-PCR (qRT-PCR) demonstrated that different isoforms of Dab1 and Fyn have tissue-specific expression patterns, and sDab1 and sFyn-B display similar temporal expression characteristics in the developing porcine cerebral cortex and cerebellum. Both sDab1 isoforms function as nucleocytoplasmic shuttling proteins. It was further shown that sFyn phosphorylates sDab1 at tyrosyl residues (Tyr) 185, 198/200 and 232, whereas sDab1-Li was phosphorylated at Tyr 185 and Tyr 197 (corresponding to Y232 in sDab1) in vitro.
Conclusions: Alternative splicing generates natural sDab1-Li that only carries Y185 and Y197 (corresponding to Y232 in sDab1) sites, which can be phosphorylated by Fyn in vitro. sDab1-Li is an isoform that is highly expressed in peripheral organs. Both isoforms are suggested to be nucleocytoplasmic shuttling proteins. Our results imply that the short splice form sDab1-Li might regulate cellular responses to different cell signals by acting as a dominant negative form against the full length sDab1 variant and that both isoforms might serve different signaling functions in different tissues.
Figures




Similar articles
-
Disabled-1 alternative splicing in human fetal retina and neural tumors.PLoS One. 2011;6(12):e28579. doi: 10.1371/journal.pone.0028579. Epub 2011 Dec 6. PLoS One. 2011. PMID: 22163036 Free PMC article.
-
Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development.Curr Biol. 2003 Jan 8;13(1):9-17. doi: 10.1016/s0960-9822(02)01397-0. Curr Biol. 2003. PMID: 12526739
-
Apolipoprotein E receptors are required for reelin-induced proteasomal degradation of the neuronal adaptor protein Disabled-1.J Biol Chem. 2004 Aug 6;279(32):33471-9. doi: 10.1074/jbc.M401770200. Epub 2004 Jun 2. J Biol Chem. 2004. PMID: 15175346
-
Relative importance of the tyrosine phosphorylation sites of Disabled-1 to the transmission of Reelin signaling.Brain Res. 2009 Dec 22;1304:26-37. doi: 10.1016/j.brainres.2009.09.087. Epub 2009 Sep 29. Brain Res. 2009. PMID: 19796633
-
Small and large intestine express a truncated Dab1 isoform that assembles in cell-cell junctions and co-localizes with proteins involved in endocytosis.Biochim Biophys Acta Biomembr. 2018 May;1860(5):1231-1241. doi: 10.1016/j.bbamem.2018.02.014. Epub 2018 Feb 19. Biochim Biophys Acta Biomembr. 2018. PMID: 29470947
Cited by
-
Quantification of type II procollagen splice forms using alternative transcript-qPCR (AT-qPCR).Matrix Biol. 2012 Sep-Oct;31(7-8):412-20. doi: 10.1016/j.matbio.2012.08.002. Epub 2012 Sep 10. Matrix Biol. 2012. PMID: 22974592 Free PMC article.
-
New Molecular Markers Involved in Regulation of Ovarian Granulosa Cell Morphogenesis, Development and Differentiation during Short-Term Primary In Vitro Culture-Transcriptomic and Histochemical Study Based on Ovaries and Individual Separated Follicles.Int J Mol Sci. 2019 Aug 15;20(16):3966. doi: 10.3390/ijms20163966. Int J Mol Sci. 2019. PMID: 31443152 Free PMC article.
-
Reelin-Disabled-1 signaling in neuronal migration: splicing takes the stage.Cell Mol Life Sci. 2013 Jul;70(13):2319-29. doi: 10.1007/s00018-012-1171-6. Epub 2012 Sep 28. Cell Mol Life Sci. 2013. PMID: 23052211 Free PMC article. Review.
-
Population genomic, olfactory, dietary, and gut microbiota analyses demonstrate the unique evolutionary trajectory of feral pigs.Mol Ecol. 2022 Jan;31(1):220-237. doi: 10.1111/mec.16238. Epub 2021 Oct 31. Mol Ecol. 2022. PMID: 34676935 Free PMC article.
-
Disabled-1 alternative splicing in human fetal retina and neural tumors.PLoS One. 2011;6(12):e28579. doi: 10.1371/journal.pone.0028579. Epub 2011 Dec 6. PLoS One. 2011. PMID: 22163036 Free PMC article.
References
-
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97(6):689–701. doi: 10.1016/S0092-8674(00)80782-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

