Role of taurine on acid secretion in the rat stomach
- PMID: 21294907
- PMCID: PMC3042912
- DOI: 10.1186/1423-0127-18-11
Role of taurine on acid secretion in the rat stomach
Abstract
Background: Taurine has chemical structure similar to an inhibitory neurotransmitter, γ-aminobutyric acid (GABA). Previous studies on GABA in the stomach suggest GABAergic neuron is involved in acid secretion, but the effects of taurine are poor understood.
Methods: The effects of taurine on acid secretion, signal transduction, and localization of taurinergic neurons were determined in the rat stomach using everted whole stomach, RIA kit and immunohistochemical methods.
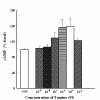
Results: We used antibodies against taurine-synthesizing enzyme, cysteine sulfuric acid decarboxylase (CSAD), and taurine. CSAD- and taurine-positive cells were found in the muscle and mucosal layers. Distributions of CSAD- and taurine-positive cells in both mucosal and muscle layers were heterogeneous in the stomach. Taurine at 10-9~10-4 M induced acid secretion, and the maximum secretion was at 10-5 M, 1.6-fold higher than the spontaneous secretion. Taurine-induced acid secretion was completely inhibited by bicuculline and atropine but not by cimetidine, proglumide, or strychnine. Atropine and tetrodotoxin (TTX) completely inhibited the acid secretion induced by low concentrations of taurine and partially inhibited induced by high concentrations. Verapamil, a calcium blocker agent, inhibited acid output elicited by taurine. We assumed all Ca2+ channels involved in the response to these secretagogues were equally affected by verapamil. Intracellular cAMP (adenosine 3', 5'-monophosphate) in the stomach significantly increased with taurine treatment in a dose-dependent manner. High correlation (r=0.859, p < 0.001) of taurine concentrations with cAMP was observed.
Conclusions: Our results demonstrated for the first time in taurine-induced acid secretion due to increase intracellular calcium may act through the A type of GABA receptors, which are mainly located on cholinergic neurons though cAMP pathway and partially on nonneuronal cells in the rat stomach.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

