Cancer cell invasion: treatment and monitoring opportunities in nanomedicine
- PMID: 21295093
- PMCID: PMC3132387
- DOI: 10.1016/j.addr.2011.01.010
Cancer cell invasion: treatment and monitoring opportunities in nanomedicine
Abstract
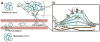
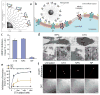
Cell invasion is an intrinsic cellular pathway whereby cells respond to extracellular stimuli to migrate through and modulate the structure of their extracellular matrix (ECM) in order to develop, repair, and protect the body's tissues. In cancer cells this process can become aberrantly regulated and lead to cancer metastasis. This cellular pathway contributes to the vast majority of cancer related fatalities, and therefore has been identified as a critical therapeutic target. Researchers have identified numerous potential molecular therapeutic targets of cancer cell invasion, yet delivery of therapies remains a major hurdle. Nanomedicine is a rapidly emerging technology which may offer a potential solution for tackling cancer metastasis by improving the specificity and potency of therapeutics delivered to invasive cancer cells. In this review we examine the biology of cancer cell invasion, its role in cancer progression and metastasis, molecular targets of cell invasion, and therapeutic inhibitors of cell invasion. We then discuss how the field of nanomedicine can be applied to monitor and treat cancer cell invasion. We aim to provide a perspective on how the advances in cancer biology and the field of nanomedicine can be combined to offer new solutions for treating cancer metastasis.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Modulating the tumor microenvironment with new therapeutic nanoparticles: A promising paradigm for tumor treatment.Med Res Rev. 2020 May;40(3):1084-1102. doi: 10.1002/med.21644. Epub 2019 Nov 11. Med Res Rev. 2020. PMID: 31709590 Review.
-
Rational design of multimodal therapeutic nanosystems for effective inhibition of tumor growth and metastasis.Acta Biomater. 2018 Sep 1;77:240-254. doi: 10.1016/j.actbio.2018.07.025. Epub 2018 Jul 20. Acta Biomater. 2018. PMID: 30012354
-
Delta-like ligand 4-targeted nanomedicine for antiangiogenic cancer therapy.Biomaterials. 2015 Feb;42:161-71. doi: 10.1016/j.biomaterials.2014.11.039. Epub 2014 Dec 16. Biomaterials. 2015. PMID: 25542804
-
Targeting invadopodia for blocking breast cancer metastasis.Drug Resist Updat. 2018 Jul;39:1-17. doi: 10.1016/j.drup.2018.05.002. Epub 2018 May 17. Drug Resist Updat. 2018. PMID: 30075834 Review.
-
Challenges and Opportunities from Basic Cancer Biology for Nanomedicine for Targeted Drug Delivery.Curr Cancer Drug Targets. 2019;19(4):257-276. doi: 10.2174/1568009618666180628160211. Curr Cancer Drug Targets. 2019. PMID: 29956629 Review.
Cited by
-
Investigation of miltefosine-model membranes interactions at the molecular level for two different PS levels modeling cancer cells.J Bioenerg Biomembr. 2024 Aug;56(4):461-473. doi: 10.1007/s10863-024-10025-y. Epub 2024 Jun 4. J Bioenerg Biomembr. 2024. PMID: 38833041 Free PMC article.
-
Current Advances in Black Phosphorus-Based Drug Delivery Systems for Cancer Therapy.Adv Sci (Weinh). 2021 Jan 15;8(5):2003033. doi: 10.1002/advs.202003033. eCollection 2021 Mar. Adv Sci (Weinh). 2021. PMID: 33717847 Free PMC article. Review.
-
Therapeutic nanosystems for oncology nanomedicine.Clin Transl Oncol. 2012 Dec;14(12):883-90. doi: 10.1007/s12094-012-0912-1. Epub 2012 Jul 27. Clin Transl Oncol. 2012. PMID: 22855194
-
Carrying epirubicin on nanoemulsion containing algae and cinnamon oils augments its apoptotic and anti-invasion effects on human colon cancer cells.Am J Transl Res. 2020 Jun 15;12(6):2463-2472. eCollection 2020. Am J Transl Res. 2020. PMID: 32655784 Free PMC article.
-
Promising Nanomedicines of Shikonin for Cancer Therapy.Int J Nanomedicine. 2023 Mar 10;18:1195-1218. doi: 10.2147/IJN.S401570. eCollection 2023. Int J Nanomedicine. 2023. PMID: 36926681 Free PMC article. Review.
References
-
- Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–24. - PubMed
-
- Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. - PubMed
-
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–58. - PubMed
-
- Weber GF. Molecular mechanisms of metastasis. Cancer Lett. 2008;270:181–90. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

