ΔNp63α is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis
- PMID: 21295273
- PMCID: PMC4373450
- DOI: 10.1016/j.stem.2010.12.009
ΔNp63α is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis
Abstract
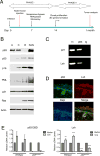
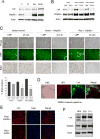
The p53 homolog p63 is essential for development, yet its role in cancer is not clear. We discovered that p63 deficiency evokes the tumor-suppressive mechanism of cellular senescence, causing a striking absence of stratified epithelia such as the skin. Here we identify the predominant p63 isoform, ΔNp63α, as a protein that bypasses oncogene-induced senescence to drive tumorigenesis in vivo. Interestingly, bypass of senescence promotes stem-like proliferation and maintains survival of the keratin 15-positive stem cell population. Furthermore, we identify the chromatin-remodeling protein Lsh as a new target of ΔNp63α that is an essential mediator of senescence bypass. These findings indicate that ΔNp63α is an oncogene that cooperates with Ras to promote tumor-initiating stem-like proliferation and suggest that Lsh-mediated chromatin-remodeling events are critical to this process.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
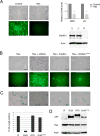
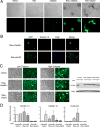
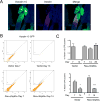

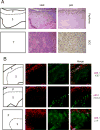
References
-
- Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. - PubMed
-
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, Pawson T, Muthuswamy SK. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–1245. - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

