Yeast ornithine decarboxylase and antizyme form a 1:1 complex in vitro: purification and characterization of the inhibitory complex
- PMID: 21295540
- PMCID: PMC3064986
- DOI: 10.1016/j.bbrc.2011.01.113
Yeast ornithine decarboxylase and antizyme form a 1:1 complex in vitro: purification and characterization of the inhibitory complex
Abstract
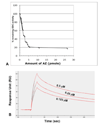
Saccharomyces cerevisiae antizyme (AZ) resembles mammalian AZ in its mode of synthesis by translational frameshifting and its ability to inhibit and facilitate the degradation of ornithine decarboxylase (ODC). Despite many studies on the interaction of AZ and ODC, the ODC:AZ complex has not been purified from any source and thus clear information about the stoichiometry of the complex is still lacking. In this study we have studied the yeast antizyme protein and the ODC:AZ complex. The far UV CD spectrum of the full-length antizyme shows that the yeast protein consists of 51% β-sheet, 19% α-helix, and 24% coils. Surface plasmon resonance analyses show that the association constant (K(A)) between yeast AZ and yeast ODC is 6×10(7) (M(-1)). Using purified His-tagged AZ as a binding partner, we have purified the ODC:AZ inhibitory complex. The isolated complex has no ODC activity. The molecular weight of the complex is 90 kDa, which indicates a one to one stoichiometric binding of AZ and ODC in vitro. Comparison of the circular dichroism (CD) spectra of the two individual proteins and of the ODC:AZ complex shows a change in the secondary structure in the complex.
Published by Elsevier Inc.
Figures



References
-
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. - PubMed
-
- Cohen SS. A Guide to the Polyamines. New York: Oxford Univ. Press; 1998. pp. 1–595.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

