Multiple treatment cycles of liposome-encapsulated adenoviral RIP-TK gene therapy effectively ablate human pancreatic cancer cells in SCID mice
- PMID: 21295812
- PMCID: PMC3072061
- DOI: 10.1016/j.surg.2010.11.014
Multiple treatment cycles of liposome-encapsulated adenoviral RIP-TK gene therapy effectively ablate human pancreatic cancer cells in SCID mice
Abstract
Background: Adenoviral gene therapy has been applied widely for cancer therapy; however, transient gene expression as result of humoral immunoneutralization response to adenovirus limits its effect. The purpose of this study is to determine whether DOTAP:cholesterol liposome could shield adenovirus from neutralizing antibody and permit the use of multiple cycles of intravenous liposome encapsulated serotype 5 adenoviral rat insulin promoter directed thymidine kinase (L-A-5-RIP-TK) with ganciclovir (GCV) to enhance its effect.
Methods: The effect of multiple cycles of systemic L-A-5-RIP-TK/GCV therapy was evaluated in grouped PANC-1 SCID mice treated with different numbers of cycles. Humoral immune response to A-5-RIP-TK or L-A-5-RIP-TK was assessed using C57/B6J mice challenged with adenovirus or liposome adenovirus complex.
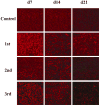
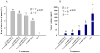
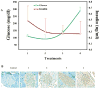
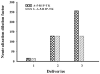
Results: The minimal residual tumor burden (3.2 ± 0.6 mm(3)) and greatest survival time (153.0 ± 6 days) were obtained in the mice receiving 4 and 3 cycles of therapy, respectively. Toxicity to islet cells associated with RIP-TK/GCV therapy was observed after 4 cycles. DOTAP:chol-encapsulated adenovectors were able to protect adenovectors from the neutralization of high titer of anti-adenoviral antibodies induced by itself.
Conclusion: Multiple treatment cycles of L-A-5-RIP-TK/GCV ablate human PANC-1 cells effectively in SCID mice; however, the mice become diabetic and have substantial mortality after the 4th cycle. Liposome-encapsulated adenovirus is functionally resistant to the neutralizing effects of anti-adenoviral antibodies, suggesting feasibility of multiple cycles of therapy. Liposome encapsulation of the adenovirus may be a promising strategy for repeated delivery of systemic adenoviral gene therapy.
Copyright © 2011 Mosby, Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: There is no conflict of interest on this research.
Figures

References
-
- Sener SF, Fremgen A, Menck HR, Winchester DP. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. - PubMed
-
- Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, et al. Surgical treatment of pancreatic adenocarcinoma; actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–58. - PubMed
-
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–58. - PubMed
-
- Prince HM, Dessureault S, Gallinger S, Krajden M, Sutherland DR, Addison C, et al. Efficient adenovirus-mediated gene expression in malignant human plasma cells: relative lymphoid cell resistance. Exp Hematol. 1998;26:27–36. - PubMed
-
- Pearson AS, Koch PE, Atkinson N, Xiong M, Finberg RW, Roth JA, et al. Factors limiting adenovirus-mediated gene transfer into human lung and pancreatic cancer cell lines. Clin Cancer Res. 1999;5:4208–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

