The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles
- PMID: 21295849
- PMCID: PMC3055170
- DOI: 10.1016/j.biomaterials.2011.01.021
The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles
Abstract
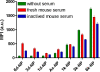
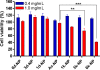
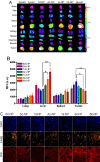
To systematically elucidate the effect of surface charge on the cellular uptake and in vivo fate of PEG-oligocholic acid based micellar nanoparticles (NPs), the distal PEG termini of monomeric PEG-oligocholic acid dendrimers (telodendrimers) are each derivatized with different number (n = 0, 1, 3 and 6) of anionic aspartic acids (negative charge) or cationic lysines (positive charge). Under aqueous condition, these telodendrimers self-assemble to form a series of micellar NPs with various surface charges, but with similar particle sizes. NPs with high surface charge, either positive or negative, were taken up more efficiently by RAW 264.7 murine macrophages after opsonization in fresh mouse serum. Mechanistic studies of cellular uptake of NPs indicated that several distinct endocytic pathways (e.g., clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis) were involved in the cellular uptake process. After their cellular uptake, the majority of NPs were found to localize in the lysosome. Positively charged NPs exhibited dose-dependent hemolytic activities and cytotoxicities against RAW 264.7 cells proportional to the positive surface charge densities; whereas negatively charged NPs did not show obvious hemolytic and cytotoxic properties. In vivo biodistribution studies demonstrated that undesirable liver uptake was very high for highly positively or negatively charged NPs, which is likely due to active phagocytosis by macrophages (Kupffer cells) in the liver. In contrast, liver uptake was very low but tumor uptake was very high when the surface charge of NPs was slightly negative. Based on these studies, we can conclude that slightly negative charge may be introduced to the NPs surface to reduce the undesirable clearance by the reticuloendothelial system (RES) such as liver, improve the blood compatibility, thus deliver the anti-cancer drugs more efficiently to the tumor sites.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures







References
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. - PubMed
-
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–82. - PubMed
-
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–3. - PubMed
-
- Zahr AS, Davis CA, Pishko MV. Macrophage uptake of core-shell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22(19):8178–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

