Regulation of Bach2 by the aryl hydrocarbon receptor as a mechanism for suppression of B-cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin
- PMID: 21296099
- PMCID: PMC3106294
- DOI: 10.1016/j.taap.2011.01.020
Regulation of Bach2 by the aryl hydrocarbon receptor as a mechanism for suppression of B-cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin
Abstract
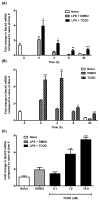
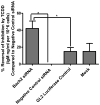
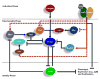
Exposure to the aryl hydrocarbon receptor (AHR) agonist, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters B-cell differentiation and suppresses antibody production. Previous genomic studies in mouse B cells identified Bach2 as a direct target of the AHR. Bach2 is known to repress expression of Prdm1, a key transcription factor involved in B-cell differentiation, by binding to Maf elements (MAREs) in the regulatory regions of the gene. Chromatin immunoprecipitation followed by quantitative PCR in TCDD-treated lipopolysaccharide (LPS)-activated B cells showed increased binding of the AHR within the first intron in the Bach2 gene. The binding was further confirmed by electrophoretic mobility shift assay (EMSA). TCDD also induced expression of Bach2 in activated as well as resting B cells from 2 to 24h post-treatment in a time- and concentration-dependent manner. Expression of Prdm1 was decreased by TCDD at 24h and was consistent with repression by Bach2. Increased DNA binding activity to the intron 5 MARE with increasing TCDD concentrations was observed by EMSA. Supershifts identified the presence of Bach2 in the DNA binding complex associated with the intron 5 MARE of Prdm1. Functional validation of the role of Bach2 in the suppression of B-cell differentiation by TCDD was performed using RNA interference (RNAi). Knockdown of Bach2 showed approximately 40% reversal in the TCDD-induced suppression of IgM secretion when compared to controls. The results suggest that the transcriptional regulation of Bach2 by the AHR is one of the mechanisms involved in the suppression of B-cell differentiation by TCDD.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Russell S. Thomas acknowledges that he has received grant funding from The Dow Chemical Company for work in a different area.
Figures

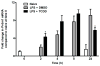




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

