The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis
- PMID: 21296189
- PMCID: PMC3075374
- DOI: 10.1016/j.mito.2011.01.010
The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis
Abstract
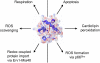
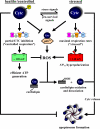
Cytochrome c (Cytc) is essential in mitochondrial electron transport and intrinsic type II apoptosis. Mammalian Cytc also scavenges reactive oxygen species (ROS) under healthy conditions, produces ROS with the co-factor p66(Shc), and oxidizes cardiolipin during apoptosis. The recent finding that Cytc is phosphorylated in vivo underpins a model for the pivotal role of Cytc regulation in making life and death decisions. An apoptotic sequence of events is proposed involving changes in Cytc phosphorylation, increased ROS via increased mitochondrial membrane potentials or the p66(Shc) pathway, and oxidation of cardiolipin by Cytc followed by its release from the mitochondria. Cytc regulation in respiration and cell death is discussed in a human disease context including neurodegenerative and cardiovascular diseases, cancer, and sepsis.
Copyright © 2011 Elsevier B.V. and Mitochondria Research Society. All rights reserved.
Figures
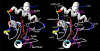

References
-
- Acin-Perez R, Bayona-Bafaluy MP, Bueno M, Machicado C, Fernandez-Silva P, Perez-Martos A, Montoya J, Lopez-Perez MJ, Sancho J, Enriquez JA. An intragenic suppressor in the cytochrome c oxidase I gene of mouse mitochondrial DNA. Hum Mol Genet. 2003;12:329–339. - PubMed
-
- Allen S, Balabanidou V, Sideris DP, Lisowsky T, Tokatlidis K. Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J Mol Biol. 2005;353:937–944. - PubMed
-
- Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. - PubMed
-
- Arnold S, Kadenbach B. The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 1999;443:105–108. - PubMed
-
- Backus M, Piwnica-Worms D, Hockett D, Kronauge J, Lieberman M, Ingram P, LeFurgey A. Microprobe analysis of Tc-MIBI in heart cells: calculation of mitochondrial membrane potential. Am J Physiol. 1993;265:C178–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

