Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release
- PMID: 21296500
- PMCID: PMC3108328
- DOI: 10.1016/j.pain.2010.12.034
Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release
Abstract
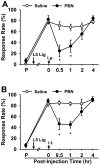
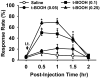
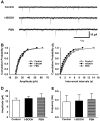
Although both a loss of spinal inhibitory neurotransmission and the involvement of oxidative stress have been regarded as important mechanisms in the pathogenesis of pain, the relationship between these 2 mechanisms has not been studied. To determine whether reactive oxygen species (ROS) involvement in pain mechanisms is related to the diminished inhibitory transmission in the substantia gelatinosa (SG) of the spinal dorsal horn, behavioral studies and whole-cell recordings were performed in FVB/NJ mice. Neuropathic pain was induced by a tight ligation of the L5 spinal nerve (SNL). Pain behaviors in the affected foot were assessed by behavioral testing for mechanical hyperalgesia. Pain behaviors developed by 3 days and lasted more than 8 weeks. Both systemic and intrathecal administration of an ROS scavenger, phenyl-N-tert-butylnitrone (PBN), temporarily reversed mechanical hyperalgesia up to 2 hours, 1 week after SNL. In nonligated mice, an intrathecal injection of an ROS donor, tert-butyl hydroperoxide (t-BOOH), dose-dependently induced mechanical hyperalgesia for 1.5 hours. In whole-cell voltage clamp recordings of SG neurons, perfusion with t-BOOH significantly decreased the frequency of mIPSCs, and this effect was reversed by PBN. Furthermore, t-BOOH decreased the frequency of GABA(A) receptor-mediated mIPSCs without altering their amplitudes but did not affect glycine receptor-mediated mIPSCs. In SNL mice, mIPSC frequency in SG neurons was significantly reduced as compared with that of normal mice, which was restored by PBN. The antihyperalgesic effect of PBN on mechanical hyperalgesia was attenuated by intrathecal bicuculline, a GABA(A) receptor blocker. Our results indicate that the increased ROS in spinal cord may induce pain by reducing GABA inhibitory influence on SG neurons that are involved in pain transmission.
Copyright © 2010 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest through financial or other relationships.
Figures





References
-
- Alia M, Ramos S, Mateos R, Bravo L, Goya L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J Biochem Mol Toxicol. 2005;19:119–28. - PubMed
-
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
-
- Chen Q, Pan HL. Signaling mechanisms of angiotensin II-induced attenuation of GABAergic input to hypothalamic presympathetic neurons. J Neurophysiol. 2007;97:3279–87. - PubMed
-
- Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15:96–103. - PubMed
-
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

