Cone photoreceptor abnormalities correlate with vision loss in patients with Stargardt disease
- PMID: 21296825
- PMCID: PMC3109028
- DOI: 10.1167/iovs.10-6538
Cone photoreceptor abnormalities correlate with vision loss in patients with Stargardt disease
Abstract
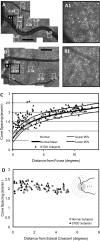
PURPOSE. To study the relationship between macular cone structure, fundus autofluorescence (AF), and visual function in patients with Stargardt disease (STGD). METHODS. High-resolution images of the macula were obtained with adaptive optics scanning laser ophthalmoscopy (AOSLO) and spectral domain optical coherence tomography in 12 patients with STGD and 27 age-matched healthy subjects. Measures of retinal structure and AF were correlated with visual function, including best-corrected visual acuity, color vision, kinetic and static perimetry, fundus-guided microperimetry, and full-field electroretinography. Mutation analysis of the ABCA4 gene was completed in all patients. RESULTS. Patients were 15 to 55 years old, and visual acuity ranged from 20/25-20/320. Central scotomas were present in all patients, although the fovea was spared in three patients. The earliest cone spacing abnormalities were observed in regions of homogeneous AF, normal visual function, and normal outer retinal structure. Outer retinal structure and AF were most normal near the optic disc. Longitudinal studies showed progressive increases in AF followed by reduced AF associated with losses of visual sensitivity, outer retinal layers, and cones. At least one disease-causing mutation in the ABCA4 gene was identified in 11 of 12 patients studied; 1 of 12 patients showed no disease-causing ABCA4 mutations. CONCLUSIONS. AOSLO imaging demonstrated abnormal cone spacing in regions of abnormal fundus AF and reduced visual function. These findings provide support for a model of disease progression in which lipofuscin accumulation results in homogeneously increased AF with cone spacing abnormalities, followed by heterogeneously increased AF with cone loss, then reduced AF with cone and RPE cell death.
Figures






References
-
- Armstrong JD, Meyer D, Xu S, Elfervig JL. Long-term follow-up of Stargardt's disease and fundus flavimaculatus. Ophthalmology. 1998;105:448–457, discussion 457–448 - PubMed
-
- Fishman GA, Farber M, Patel BS, Derlacki DJ. Visual acuity loss in patients with Stargardt's macular dystrophy. Ophthalmology. 1987;94:809–814 - PubMed
-
- Glazer LC, Dryja TP. Understanding the etiology of Stargardt's disease. Ophthalmol Clin North Am. 2002;15:93–100, viii - PubMed
-
- Rotenstreich Y, Fishman GA, Anderson RJ. Visual acuity loss and clinical observations in a large series of patients with Stargardt disease. Ophthalmology. 2003;110:1151–1158 - PubMed
-
- Birnbach CD, Jarvelainen M, Possin DE, Milam AH. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 1994;101:1211–1219 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

