Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice
- PMID: 21296886
- PMCID: PMC3064218
- DOI: 10.1074/jbc.M110.203000
Inactivation of the mitochondrial carrier SLC25A25 (ATP-Mg2+/Pi transporter) reduces physical endurance and metabolic efficiency in mice
Abstract
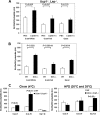
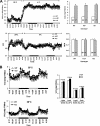
An ATP-Mg(2+/)P(i) inner mitochondrial membrane solute transporter (SLC25A25), which is induced during adaptation to cold stress in the skeletal muscle of mice with defective UCP1/brown adipose tissue thermogenesis, has been evaluated for its role in metabolic efficiency. SLC25A25 is thought to control ATP homeostasis by functioning as a Ca(2+)-regulated shuttle of ATP-Mg(2+) and P(i) across the inner mitochondrial membrane. Mice with an inactivated Slc25a25 gene have reduced metabolic efficiency as evidenced by enhanced resistance to diet-induced obesity and impaired exercise performance on a treadmill. Mouse embryo fibroblasts from Slc25a25(-/-) mice have reduced Ca(2+) flux across the endoplasmic reticulum, basal mitochondrial respiration, and ATP content. Although Slc25a25(-/-) mice are metabolically inefficient, the source of the inefficiency is not from a primary function in thermogenesis, because Slc25a25(-/-) mice maintain body temperature upon acute exposure to the cold (4 °C). Rather, the role of SLC25A25 in metabolic efficiency is most likely linked to muscle function as evidenced from the physical endurance test of mutant mice on a treadmill. Consequently, in the absence of SLC25A25 the efficiency of ATP production required for skeletal muscle function is diminished with secondary effects on adiposity. However, in the absence of UCP1-based thermogenesis, induction of Slc25a25 in mice with an intact gene may contribute to an alternative thermogenic pathway for the maintenance of body temperature during cold stress.
Figures





References
-
- Morton G. J., Cummings D. E., Baskin D. G., Barsh G. S., Schwartz M. W. (2006) Nature 443, 289–295 - PubMed
-
- Hawley J. A., Holloszy J. O. (2009) Nutr. Rev. 67, 172–178 - PubMed
-
- Zurlo F., Lillioja S., Esposito-Del Puente A., Nyomba B. L., Raz I., Saad M. F., Swinburn B. A., Knowler W. C., Bogardus C., Ravussin E. (1990) Am. J. Physiol. Endocrinol. Metab. 259, E650-E657 - PubMed
-
- Lowell B. B., Shulman G. I. (2005) Science 307, 384–387 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

