Lactate dehydrogenase A promotes communication between carbohydrate catabolism and virulence in Bacillus cereus
- PMID: 21296961
- PMCID: PMC3067659
- DOI: 10.1128/JB.00024-11
Lactate dehydrogenase A promotes communication between carbohydrate catabolism and virulence in Bacillus cereus
Abstract
The diarrheal potential of a Bacillus cereus strain is essentially dictated by the amount of secreted nonhemolytic enterotoxin (Nhe). Expression of genes encoding Nhe is regulated by several factors, including the metabolic state of the cells. To identify metabolic sensors that could promote communication between central metabolism and nhe expression, we compared four strains of the B. cereus group in terms of metabolic and nhe expression capacities. We performed growth performance measurements, metabolite analysis, and mRNA measurements of strains F4430/73, F4810/72, F837/76, and PA cultured under anoxic and fully oxic conditions. The results showed that expression levels of nhe and ldhA, which encodes lactate dehydrogenase A (LdhA), were correlated in both aerobically and anaerobically grown cells. We examined the role of LdhA in the F4430/73 strain by constructing an ldhA mutant. The ldhA mutation was more deleterious to anaerobically grown cells than to aerobically grown cells, causing growth limitation and strong deregulation of key fermentative genes. More importantly, the ldhA mutation downregulated enterotoxin gene expression under both anaerobiosis and aerobiosis, with a more pronounced effect under anaerobiosis. Therefore, LdhA was found to exert a major control on both fermentative growth and enterotoxin expression, and it is concluded that there is a direct link between fermentative metabolism and virulence in B. cereus. The data presented also provide evidence that LdhA-dependent regulation of enterotoxin gene expression is oxygen independent. This study is the first report to describe a role of a fermentative enzyme in virulence in B. cereus.
Figures
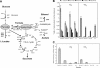


References
-
- Agaisse, H., M. Gominet, O. A. Okstad, A. B. Kolsto, and D. Lereclus. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043-1053. - PubMed
-
- Arai, K., et al. 2010. Active and inactive state structures of unliganded Lactobacillus casei allosteric L-lactate dehydrogenase. Proteins 78:681-694. - PubMed
-
- Arantes, O., and D. Lereclus. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115-119. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

