Effects of modified Phycobilin biosynthesis in the Cyanobacterium Synechococcus sp. Strain PCC 7002
- PMID: 21296968
- PMCID: PMC3067670
- DOI: 10.1128/JB.01392-10
Effects of modified Phycobilin biosynthesis in the Cyanobacterium Synechococcus sp. Strain PCC 7002
Abstract
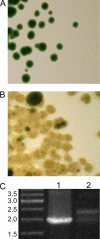
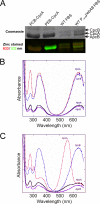
The pathway for phycocyanobilin biosynthesis in Synechococcus sp. strain PCC 7002 comprises two enzymes: heme oxygenase and phycocyanobilin synthase (PcyA). The phycobilin content of cells can be modified by overexpressing genes encoding alternative enzymes for biliverdin reduction. Overexpression of the pebAB and HY2 genes, encoding alternative ferredoxin-dependent biliverdin reductases, caused unique effects due to the overproduction of phycoerythrobilin and phytochromobilin, respectively. Colonies overexpressing pebAB became reddish brown and visually resembled strains that naturally produce phycoerythrin. This was almost exclusively due to the replacement of phycocyanobilin by phycoerythrobilin on the phycocyanin α-subunit. This phenotype was unstable, and such strains rapidly reverted to the wild-type appearance, presumably due to strong selective pressure to inactivate pebAB expression. Overproduction of phytochromobilin, synthesized by the Arabidopsis thaliana HY2 product, was tolerated much better. Cells overexpressing HY2 were only slightly less pigmented and blue-green than the wild type. Although the pcyA gene could not be inactivated in the wild type, pcyA was easily inactivated when cells expressed HY2. These results indicate that phytochromobilin can functionally substitute for phycocyanobilin in Synechococcus sp. strain PCC 7002. Although functional phycobilisomes were assembled in this strain, the overall phycobiliprotein content of cells was lower, the efficiency of energy transfer by these phycobilisomes was lower than for wild-type phycobilisomes, and the absorption cross-section of the cells was reduced relative to that of the wild type because of an increased spectral overlap of the modified phycobiliproteins with chlorophyll a. As a result, the strain producing phycobiliproteins carrying phytochromobilin grew much more slowly at low light intensity.
Figures







Similar articles
-
Attachment of noncognate chromophores to CpcA of Synechocystis sp. PCC 6803 and Synechococcus sp. PCC 7002 by heterologous expression in Escherichia coli.Biochemistry. 2011 Jun 7;50(22):4890-902. doi: 10.1021/bi200307s. Epub 2011 May 16. Biochemistry. 2011. PMID: 21553904
-
Under light limiting growth, CpcB lyase null mutants of the Cyanobacterium Synechococcus sp. PCC 7002 are capable of producing pigmented beta phycocyanin but with altered chromophore function.Biochemistry. 2008 Nov 11;47(45):11877-84. doi: 10.1021/bi702143a. Epub 2008 Oct 17. Biochemistry. 2008. PMID: 18925744
-
Adapting photosynthesis to the near-infrared: non-covalent binding of phycocyanobilin provides an extreme spectral red-shift to phycobilisome core-membrane linker from Synechococcus sp. PCC7335.Biochim Biophys Acta. 2016 Jun;1857(6):688-94. doi: 10.1016/j.bbabio.2016.03.033. Epub 2016 Apr 2. Biochim Biophys Acta. 2016. PMID: 27045046
-
Biosynthesis of phycobilins. Formation of the chromophore of phytochrome, phycocyanin and phycoerythrin.J Photochem Photobiol B. 1990 Apr 1;5(1):3-23. doi: 10.1016/1011-1344(90)85002-e. J Photochem Photobiol B. 1990. PMID: 2111391 Review.
-
Phycobiliproteins in Prochlorococcus marinus: biosynthesis of pigments and their assembly into proteins.Eur J Cell Biol. 2010 Dec;89(12):1005-10. doi: 10.1016/j.ejcb.2010.06.017. Epub 2010 Aug 17. Eur J Cell Biol. 2010. PMID: 20724022 Review.
Cited by
-
Characterization of the activities of the CpeY, CpeZ, and CpeS bilin lyases in phycoerythrin biosynthesis in Fremyella diplosiphon strain UTEX 481.J Biol Chem. 2011 Oct 14;286(41):35509-35521. doi: 10.1074/jbc.M111.284281. Epub 2011 Aug 24. J Biol Chem. 2011. PMID: 21865169 Free PMC article.
-
Characterization of Ferredoxin-Dependent Biliverdin Reductase PCYA1 Reveals the Dual Function in Retrograde Bilin Biosynthesis and Interaction With Light-Dependent Protochlorophyllide Oxidoreductase LPOR in Chlamydomonas reinhardtii.Front Plant Sci. 2018 May 23;9:676. doi: 10.3389/fpls.2018.00676. eCollection 2018. Front Plant Sci. 2018. PMID: 29875782 Free PMC article.
-
Structural and biochemical characterization of the bilin lyase CpcS from Thermosynechococcus elongatus.Biochemistry. 2013 Dec 3;52(48):8663-76. doi: 10.1021/bi401192z. Epub 2013 Nov 19. Biochemistry. 2013. PMID: 24215428 Free PMC article.
-
Phycobilisome-Deficient Strains of Synechocystis sp. PCC 6803 Have Reduced Size and Require Carbon-Limiting Conditions to Exhibit Enhanced Productivity.Plant Physiol. 2014 Jun;165(2):705-714. doi: 10.1104/pp.114.237206. Epub 2014 Apr 23. Plant Physiol. 2014. PMID: 24760817 Free PMC article.
-
Ferredoxin-dependent bilin reductases in eukaryotic algae: Ubiquity and diversity.J Plant Physiol. 2017 Oct;217:57-67. doi: 10.1016/j.jplph.2017.05.022. Epub 2017 May 31. J Plant Physiol. 2017. PMID: 28641882 Free PMC article.
References
-
- Apt, K. E., J. L. Collier, and A. R. Grossman. 1995. Evolution of the phycobiliproteins. J. Mol. Biol. 248:79-96. - PubMed
-
- Beale, S. I., and J. Cornejo. 1991. Biosynthesis of phycobilins-3(Z)-phycoerythrobilin and 3(Z)-phycocyanobilin are intermediates in the formation of 3(E)-phycocyanobilin from biliverdin-IXα. J. Biol. Chem. 266:22333-22340. - PubMed
-
- Beale, S. I., and J. Cornejo. 1991. Biosynthesis of phycobilins: 15,16-dihydrobiliverdin IXα is a partially reduced intermediate in the formation of phycobilins from biliverdin IXα. J. Biol. Chem. 266:22341-22345. - PubMed
-
- Beale, S. I., and J. Cornejo. 1991. Biosynthesis of phycobilins: ferredoxin-mediated reduction of biliverdin catalyzed by extracts of Cyanidium caldarium. J. Biol. Chem. 266:22328-22332. - PubMed
-
- Becker, A., M. Schmidt, W. Jager, and A. Puhler. 1995. New gentamicin-resistance and LacZ promoter-probe cassettes suitable for insertion mutagenesis and generation of transcriptional fusions. Gene 162:37-39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

