Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus
- PMID: 21296979
- PMCID: PMC3097030
- DOI: 10.4049/jimmunol.1000569
Involvement of CD226+ NK cells in immunopathogenesis of systemic lupus erythematosus
Abstract
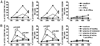
Dysfunction of immune systems, including innate and adaptive immunity, is responsible for the immunopathogenesis of systemic lupus erythematosus (SLE). NK cells are a major part of the innate immune system, and diminished populations of NK cells have been reported in SLE patients. However, the mechanisms behind this decrease and the role of NK cells in SLE pathogenesis remain poorly understood. In this study, we found that a deficiency of NK cells, especially CD226(+) NK cells, is prominent in patients with active SLE. Meanwhile, expression of the CD226 ligands CD112 and CD155 on plasmacytoid dendritic cells is observed in SLE patients; thus, activation of CD226(+) NK cells may be induced by CD226-ligand interactions. Furthermore, IFN-α, which is mainly produced by plasmacytoid dendritic cells, can mediate the activation-induced cell death of NK cells. Therefore, these processes likely contribute to the loss of NK cells in patients with active SLE. Despite the impaired cytotoxicity of peripheral NK cells in human SLE patients and mouse SLE models, we provide evidence that CD226(+) NK cells infiltrate the kidneys of predisease MRL-lpr/lpr mice. Kidney-infiltrating NK cells displayed an activated phenotype and a marked ability to produce cytotoxic granules. These results suggest that, before apoptosis, activated NK cells can infiltrate tissues and, to some extent, mediate tissue injury by producing cytotoxic granules and immunoregulatory cytokines.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


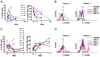





References
-
- Mills JA. Systemic lupus erythematosus. N. Engl. J. Med. 1994;330:1871–1879. - PubMed
-
- Pisetsky DS, Rönnblom L. Systemic lupus erythematosus: a matter of life and death. Arthritis Rheum. 2009;60:1567–1570. - PubMed
-
- Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. - PubMed
-
- Hess EV. Environmental lupus syndromes. Br. J. Rheumatol. 1995;34:597–599. - PubMed
-
- Chen C, Prak EL, Weigert M. Editing disease-associated auto-antibodies. Immunity. 1997;6:97–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

