Haplotype association mapping of acute lung injury in mice implicates activin a receptor, type 1
- PMID: 21297076
- PMCID: PMC3137140
- DOI: 10.1164/rccm.201006-0912OC
Haplotype association mapping of acute lung injury in mice implicates activin a receptor, type 1
Abstract
Rationale: Because acute lung injury is a sporadic disease produced by heterogeneous precipitating factors, previous genetic analyses are mainly limited to candidate gene case-control studies.
Objectives: To develop a genome-wide strategy in which single nucleotide polymorphism associations are assessed for functional consequences to survival during acute lung injury in mice.
Methods: To identify genes associated with acute lung injury, 40 inbred strains were exposed to acrolein and haplotype association mapping, microarray, and DNA-protein binding were assessed.
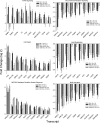
Measurements and main results: The mean survival time varied among mouse strains with polar strains differing approximately 2.5-fold. Associations were identified on chromosomes 1, 2, 4, 11, and 12. Seven genes (Acvr1, Cacnb4, Ccdc148, Galnt13, Rfwd2, Rpap2, and Tgfbr3) had single nucleotide polymorphism (SNP) associations within the gene. Because SNP associations may encompass "blocks" of associated variants, functional assessment was performed in 91 genes within ± 1 Mbp of each SNP association. Using 10% or greater allelic frequency and 10% or greater phenotype explained as threshold criteria, 16 genes were assessed by microarray and reverse real-time polymerase chain reaction. Microarray revealed several enriched pathways including transforming growth factor-β signaling. Transcripts for Acvr1, Arhgap15, Cacybp, Rfwd2, and Tgfbr3 differed between the strains with exposure and contained SNPs that could eliminate putative transcriptional factor recognition sites. Ccdc148, Fancl, and Tnn had sequence differences that could produce an amino acid substitution. Mycn and Mgat4a had a promoter SNP or 3'untranslated region SNPs, respectively. Several genes were related and encoded receptors (ACVR1, TGFBR3), transcription factors (MYCN, possibly CCDC148), and ubiquitin-proteasome (RFWD2, FANCL, CACYBP) proteins that can modulate cell signaling. An Acvr1 SNP eliminated a putative ELK1 binding site and diminished DNA-protein binding.
Conclusions: Assessment of genetic associations can be strengthened using a genetic/genomic approach. This approach identified several candidate genes, including Acvr1, associated with increased susceptibility to acute lung injury in mice.
Figures






References
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. - PubMed
-
- Traber DL, Hawkins HK, Enkhbaatar P, Cox RA, Schmalstieg FC, Zwischenberger JB, Traber LD. The role of the bronchial circulation in the acute lung injury resulting from burn and smoke inhalation. Pulm Pharmacol Ther 2007;20:163–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL075562/HL/NHLBI NIH HHS/United States
- HL084932/HL/NHLBI NIH HHS/United States
- HL085655/HL/NHLBI NIH HHS/United States
- HL075562/HL/NHLBI NIH HHS/United States
- CA134433/CA/NCI NIH HHS/United States
- HL077763/HL/NHLBI NIH HHS/United States
- CA113793/CA/NCI NIH HHS/United States
- HG003749/HG/NHGRI NIH HHS/United States
- AT003203/AT/NCCIH NIH HHS/United States
- U01 ES015675/ES/NIEHS NIH HHS/United States
- ES015675/ES/NIEHS NIH HHS/United States
- HL091938/HL/NHLBI NIH HHS/United States
- HL095397/HL/NHLBI NIH HHS/United States
- LM009662/LM/NLM NIH HHS/United States
- AT005522/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

