Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis
- PMID: 21297130
- PMCID: PMC3105488
- DOI: 10.1093/brain/awq362
Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis
Abstract
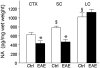
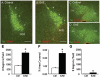
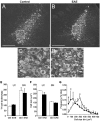
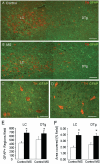
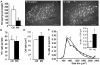
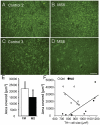
The endogenous neurotransmitter noradrenaline exerts anti-inflammatory and neuroprotective effects in vitro and in vivo. Several studies report that noradrenaline levels are altered in the central nervous system of patients with multiple sclerosis and rodents with experimental autoimmune encephalomyelitis, which could contribute to pathology. Since the major source of noradrenaline are neurons in the locus coeruleus, we hypothesized that alterations in noradrenaline levels are a consequence of stress or damage to locus coeruleus neurons. In C57BL/6 mice immunized with myelin oligodendrocyte glycoprotein peptide 35-55 to develop chronic disease, cortical and spinal cord levels of noradrenaline were significantly reduced versus control mice. Immunohistochemical staining revealed increased astrocyte activation in the ventral portion of the locus coeruleus in immunized mice. The immunized mice showed neuronal damage in the locus coeruleus detected by a reduction of average cell size of tyrosine hydroxylase stained neurons. Analysis of the locus coeruleus of multiple sclerosis and control brains showed a significant increase in astrocyte activation, a reduction in noradrenaline levels, and neuronal stress indicated by hypertrophy of tyrosine hydroxylase stained cell bodies. However, the magnitude of these changes was not correlated with extent of demyelination or of cellular infiltrates. Together these findings demonstrate the presence of inflammation and neuronal stress in multiple sclerosis as well as in experimental autoimmune encephalomyelitis. Since reduced noradrenaline levels could be permissive for increased inflammation and neuronal damage, these results suggest that methods to raise noradrenaline levels or increase locus coeruleus function may be of benefit in treating multiple sclerosis.
Figures
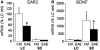
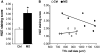
Comment in
-
Multiple sclerosis: Noradrenaline deficits suggest novel MS treatments.Nat Rev Neurol. 2011 Apr;7(4):184. doi: 10.1038/nrneurol.2011.28. Nat Rev Neurol. 2011. PMID: 21468112 No abstract available.
Similar articles
-
Role of catalpol in ameliorating the pathogenesis of experimental autoimmune encephalomyelitis by increasing the level of noradrenaline in the locus coeruleus.Mol Med Rep. 2018 Mar;17(3):4163-4172. doi: 10.3892/mmr.2018.8378. Epub 2018 Jan 5. Mol Med Rep. 2018. PMID: 29328415 Free PMC article.
-
The vincamine derivative vindeburnol provides benefit in a mouse model of multiple sclerosis: effects on the Locus coeruleus.J Neurochem. 2012 Apr;121(2):206-16. doi: 10.1111/j.1471-4159.2012.07673.x. Epub 2012 Feb 17. J Neurochem. 2012. PMID: 22288774
-
Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: noradrenergic signaling system.J Neurochem. 2016 Oct;139 Suppl 2:154-178. doi: 10.1111/jnc.13447. Epub 2016 Mar 10. J Neurochem. 2016. PMID: 26968403 Review.
-
Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway.Brain. 2011 Mar;134(Pt 3):678-92. doi: 10.1093/brain/awq386. Brain. 2011. PMID: 21354971
-
Noradrenaline in Alzheimer's Disease: A New Potential Therapeutic Target.Int J Mol Sci. 2022 May 30;23(11):6143. doi: 10.3390/ijms23116143. Int J Mol Sci. 2022. PMID: 35682822 Free PMC article. Review.
Cited by
-
Dopaminergic Therapeutics in Multiple Sclerosis: Focus on Th17-Cell Functions.J Neuroimmune Pharmacol. 2020 Mar;15(1):37-47. doi: 10.1007/s11481-019-09852-3. Epub 2019 Apr 23. J Neuroimmune Pharmacol. 2020. PMID: 31011885 Review.
-
Chemogenetic activation of locus coeruleus neurons ameliorates the severity of multiple sclerosis.J Neuroinflammation. 2023 Sep 1;20(1):198. doi: 10.1186/s12974-023-02865-z. J Neuroinflammation. 2023. PMID: 37658434 Free PMC article.
-
Gray matter sodium accumulation and atrophy in relapsing-remitting multiple sclerosis correspond to neurotransmitter maps.Eur Radiol. 2025 Jul 31. doi: 10.1007/s00330-025-11892-8. Online ahead of print. Eur Radiol. 2025. PMID: 40739057
-
Sympathetic cardiovascular and sudomotor functions are frequently affected in early multiple sclerosis.Clin Auton Res. 2016 Dec;26(6):385-393. doi: 10.1007/s10286-016-0370-x. Epub 2016 Jul 22. Clin Auton Res. 2016. PMID: 27448576
-
Neuroprotective actions of norepinephrine in neurological diseases.Pflugers Arch. 2024 Nov;476(11):1703-1725. doi: 10.1007/s00424-024-02999-w. Epub 2024 Aug 13. Pflugers Arch. 2024. PMID: 39136758 Review.
References
-
- Adolfsson R, Gottfries CG, Roos BE, Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry. 1979;135:216–23. - PubMed
-
- Barkhatova VP, Zavalishin IA, Askarova LS, Shavratskii VK, Demina EG. Changes in neurotransmitters in multiple sclerosis. Neurosci Behav Physiol. 1998;28:341–4. - PubMed
-
- Benarroch EE. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology. 2009;73:1699–704. - PubMed
-
- Benveniste EN, Huneycutt BS, Shrikant P, Ballestas ME. Second messenger systems in the regulation of cytokines and adhesion molecules in the central nervous system. Brain Behav Immun. 1995;9:304–14. - PubMed
-
- Bondareff W, Mountjoy CQ, Roth M, Rossor MN, Iversen LL, Reynolds GP, et al. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1:256–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

