An experimental and theoretical analysis of molecular separations by diffusion through ultrathin nanoporous membranes
- PMID: 21297879
- PMCID: PMC3032943
- DOI: 10.1016/j.memsci.2010.11.056
An experimental and theoretical analysis of molecular separations by diffusion through ultrathin nanoporous membranes
Abstract
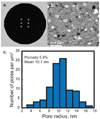
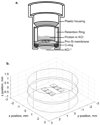
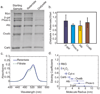
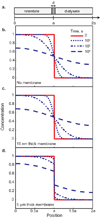
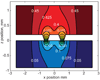
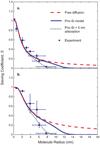
Diffusion based separations are essential for laboratory and clinical dialysis processes. New molecularly thin nanoporous membranes may improve the rate and quality of separations achievable by these processes. In this work we have performed protein and small molecule separations with 15 nm thick porous nanocrystalline silicon (pnc-Si) membranes and compared the results to 1- and 3- dimensional models of diffusion through ultrathin membranes. The models predict the amount of resistance contributed by the membrane by using pore characteristics obtained by direct inspection of pnc-Si membranes in transmission electron micrographs. The theoretical results indicate that molecularly thin membranes are expected to enable higher resolution separations at times before equilibrium compared to thicker membranes with the same pore diameters and porosities. We also explored the impact of experimental parameters such as porosity, pore distribution, diffusion time, and chamber size on the sieving characteristics. Experimental results are found to be in good agreement with the theory, and ultrathin membranes are shown to impart little overall resistance to the diffusion of molecules smaller than the physical pore size cutoff. The largest molecules tested experience more hindrance than expected from simulations indicating that factors not incorporated in the models, such as molecule shape, electrostatic repulsion, and adsorption to pore walls, are likely important.
Figures



References
-
- Luque de Castro MD, Priego Capote F, Sánchez Ávila N. Is dialysis alive as a membrane-based separations technique? TrAC, Trends Anal. Chem. 2008;27:315.
-
- Fissell WH, Humes HD, Fleischman AJ, Roy S. Dialysis and nanotechnology: now, 10 years, or never? Blood Purif. 2007;25:12. - PubMed
-
- Mochizuki S, Zydney AL. Theoretical analysis of pore size distribution effects on membrane transport. J. Mem. Sci. 1993;82:211.
-
- Vanholder R, Glorieux G, Van Biesen W. Advantages of new hemodialysis membranes and equipment. Nephron Clin. Pract. 2010;114:c165. - PubMed
-
- Jadoul M. Dialysis-related amyloidosis: importance of biocompatibility and age. Nephrol. Dial. Transpl. 1998;13:61. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
