Effects of asymmetric dimethylarginine on bovine retinal capillary endothelial cell proliferation, reactive oxygen species production, permeability, intercellular adhesion molecule-1, and occludin expression
- PMID: 21297899
- PMCID: PMC3033436
Effects of asymmetric dimethylarginine on bovine retinal capillary endothelial cell proliferation, reactive oxygen species production, permeability, intercellular adhesion molecule-1, and occludin expression
Abstract
Purpose: Asymmetric dimethylarginine (ADMA), an endogenous competitive inhibitor of nitric oxide synthase, is associated with impaired endothelial dysfunction, such as chronic heart failure, hypertension, diabetes, and pulmonary hypertension. The effects of ADMA on cell proliferation, reactive oxygen species (ROS) production, cell permeability, intercellular adhesion molecule-1 (ICAM-1), and tight-junction protein occludin levels in bovine retinal capillary endothelial cells (BRCECs) were investigated.
Methods: A cell proliferation assay was performed using the novel tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium and an electron coupling reagent. Intracellular ROS levels were determined using the fluorescent probe CM-H(2)DCFDA. Horseradish peroxidase was used for a permeability assay. ICAM-1 and tight-junction protein occludin were assessed by western blotting and quantitative real-time PCR.
Results: Cell proliferation was significantly inhibited by ADMA. ADMA increased intracellular ROS generation in BRCECs. The increased ROS production induced by ADMA was markedly inhibited by the angiotensin II receptor-blocker telmisartan, the angiotensin-converting enzyme inhibitor benazepril, the reduced form of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase inhibitor diphenyliodonium (DPI), or the antioxidant and free-radical scavenger N-acetyl-L-cysteine (NAC). ADMA significantly increased horseradish peroxidase (HRP) permeability in BRCECs. Benazepril, telmisartan, DPI, and NAC downregulated cell permeability. ADMA markedly upregulated ICAM-1 expression in BRCECs, which were downregulated by telmisartan, DPI, and NAC. ADMA significantly downregulated occludin expression in BRCECs. Benazepril and telmisartan upregulated occludin expression in BRCECs exposed to ADMA.
Conclusions: Our results provide the first reported evidence that ADMA has potent adverse effects on cell proliferation, intracellular ROS generation, cell permeability, levels of ICAM-1, and the tight-junction protein occludin. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and antioxidants are effective inhibitors of the adverse effects of ADMA.
Figures




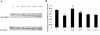
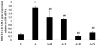
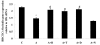
References
-
- Leiper J, Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542–8. - PubMed
-
- Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. - PubMed
-
- Böger RH, Bode-Böger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–7. - PubMed
-
- Fard A, Tuck CH, Donis JA, Sciacca R, Di Tullio MR, Wu HD, Bryant TA, Chen NT, Torres-Tamayo M, Ramasamy R, Berglund L, Ginsberg HN, Homma S, Cannon PJ. Acute elevations of plasma asymmetric dimethylarginine and impaired endothelial function in response to a high-fat meal in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol. 2000;20:2039–44. - PubMed
-
- Chen Y, Xu X, Sheng M, Zhang X, Gu Q, Zheng Z. PRMT-1 and DDAHs-induced ADMA upregulation is involved in ROS- and RAS-mediated diabetic retinopathy. Exp Eye Res. 2009;89:1028–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
