Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention
- PMID: 21297906
- PMCID: PMC3022070
- DOI: 10.3164/jcbn.11-006FR
Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention
Abstract
There is considerable interest in the role that mammalian heme peroxidase enzymes, primarily myeloperoxidase, eosinophil peroxidase and lactoperoxidase, may play in a wide range of human pathologies. This has been sparked by rapid developments in our understanding of the basic biochemistry of these enzymes, a greater understanding of the basic chemistry and biochemistry of the oxidants formed by these species, the development of biomarkers that can be used damage induced by these oxidants in vivo, and the recent identification of a number of compounds that show promise as inhibitors of these enzymes. Such compounds offer the possibility of modulating damage in a number of human pathologies. This reviews recent developments in our understanding of the biochemistry of myeloperoxidase, the oxidants that this enzyme generates, and the use of inhibitors to inhibit such damage.
Keywords: chloramines; hypochlorous acid; myeloperoxidase; neutrophil; protein oxidation.
Figures
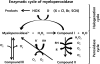
References
-
- Babior BM. The respiratory burst oxidase. TIBS. 1987;12:241–243.
-
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. - PubMed
-
- Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. - PubMed
-
- Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962;96:465–467. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

