Regulation of human neurotropic JC virus replication by alternative splicing factor SF2/ASF in glial cells
- PMID: 21297941
- PMCID: PMC3031499
- DOI: 10.1371/journal.pone.0014630
Regulation of human neurotropic JC virus replication by alternative splicing factor SF2/ASF in glial cells
Abstract
Background: The human neurotropic virus, JC virus (JCV), is the etiologic agent of the fatal demyelinating disease of the central nervous system, Progressive Multifocal Leukoencephalopathy (PML) that is seen primarily in immunodeficient individuals. Productive infection of JCV occurs only in glial cells, and this restriction is, to a great extent, due to the activation of the viral promoter that has cell type-specific characteristics. Earlier studies led to the hypothesis that glial-specific activation of the JCV promoter is mediated through positive and negative transcription factors that control reactivation of the JCV genome under normal physiological conditions and suppress its activation in non-glial cells.
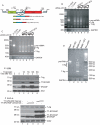
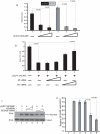
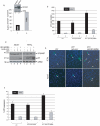
Methodology/principal findings: Using a variety of virological and molecular biological approaches, we demonstrate that the alternative splicing factor SF2/ASF has the capacity to exert a negative effect on transcription of the JCV promoter in glial cells through direct association with a specific DNA sequence within the viral enhancer/promoter region. Our results show that down-regulation of SF2/ASF in fetal and adult glial cells increases the level of JCV gene expression and its replication indicating that negative regulation of the JCV promoter by SF2/ASF may control reactivation of JCV replication in brain.
Conclusions/significance: Our results establish a new regulatory role for SF2/ASF in controlling gene expression at the transcriptional level.
Conflict of interest statement
Figures




References
-
- Weber T. Progressive Multifocal Leukoencephalopathy. Neurol Clin. 2008;26:833–854. - PubMed
-
- Imperiale MJ, Major EO. Polyomaviruses. In: Knipe DM, Howley PM, editors. Philadelphia: Lippincott, Williams & Wilkins; 2007. pp. 2263–2298. Fields Virology, 5th edition.
-
- Berger JR, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirology. 1995;1:5–18. - PubMed
-
- Safak M, Major E, Khalili K. Human polyomavirus, JC virus, and progressive multifocal encephalopathy. In: Howard IG, Gendelman E, Everall Ian Paul , Lipton StuartA., Swindells Susan, editors. The Neurology of AIDS. New York: Oxford University Press; 2005. pp. 461–474.
-
- Miller JR, Barrett RE, Britton CB, Tapper ML, Bahr GS, et al. Progressive multifocal leukoencephalopathy in a male homosexual with T-cell immune deficiency. N Engl J Med. 1982;307(23):1436–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

