CDK11(p58) is required for centriole duplication and Plk4 recruitment to mitotic centrosomes
- PMID: 21297952
- PMCID: PMC3031510
- DOI: 10.1371/journal.pone.0014600
CDK11(p58) is required for centriole duplication and Plk4 recruitment to mitotic centrosomes
Abstract
Background: CDK11(p58) is a mitotic protein kinase, which has been shown to be required for different mitotic events such as centrosome maturation, chromatid cohesion and cytokinesis.
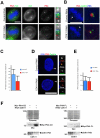
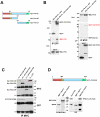
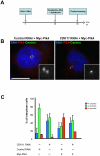
Methodology/principal findings: In addition to these previously described roles, our study shows that CDK11(p58) inhibition induces a failure in the centriole duplication process in different human cell lines. We propose that this effect is mediated by the defective centrosomal recruitment of proteins at the onset of mitosis. Indeed, Plk4 protein kinase and the centrosomal protein Cep192, which are key components of the centriole duplication machinery, showed reduced levels at centrosomes of mitotic CDK11-depleted cells. CDK11(p58), which accumulates only in the vicinity of mitotic centrosomes, directly interacts with the centriole-associated protein kinase Plk4 that regulates centriole number in cells. In addition, we show that centriole from CDK11 defective cells are not able to be over duplicated following Plk4 overexpression.
Conclusion/significance: We thus propose that CDK11 is required for centriole duplication by two non-mutually-exclusive mechanisms. On one hand, the observed duplication defect could be caused indirectly by a failure of the centrosome to fully maturate during mitosis. On the other hand, CDK11(p58) could also directly regulate key centriole components such as Plk4 during mitosis to trigger essential mitotic centriole modifications, required for centriole duplication during subsequent interphase.
Conflict of interest statement
Figures


References
-
- Kellogg DR, Moritz M, Alberts BM. The centrosome and cellular organization. Annu Rev Biochem. 1994;63:639–674. - PubMed
-
- Nigg EA. Centrosome duplication: of rules and licenses. Trends Cell Biol. 2007;17:215–221. - PubMed
-
- Nigg EA. Centrosome aberrations: cause or consequence of cancer progression? Nat Rev Cancer. 2002;2:815–825. - PubMed
-
- Nigg EA. Origins and consequences of centrosome aberrations in human cancers. Int J Cancer. 2006;119:2717–2723. - PubMed
-
- Delattre M, Leidel S, Wani K, Baumer K, Bamat J, et al. Centriolar SAS-5 is required for centrosome duplication in C. elegans. Nat Cell Biol. 2004;6:656–664. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

