Abnormal kinetochore-generated pulling forces from expressing a N-terminally modified Hec1
- PMID: 21297979
- PMCID: PMC3030568
- DOI: 10.1371/journal.pone.0016307
Abnormal kinetochore-generated pulling forces from expressing a N-terminally modified Hec1
Abstract
Background: Highly Expressed in Cancer protein 1 (Hec1) is a constituent of the Ndc80 complex, a kinetochore component that has been shown to have a fundamental role in stable kinetochore-microtubule attachment, chromosome alignment and spindle checkpoint activation at mitosis. HEC1 RNA is found up-regulated in several cancer cells, suggesting a role for HEC1 deregulation in cancer. In light of this, we have investigated the consequences of experimentally-driven Hec1 expression on mitosis and chromosome segregation in an inducible expression system from human cells.
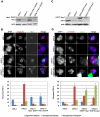
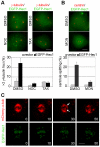
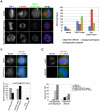
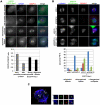
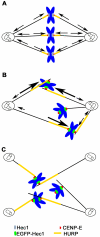
Methodology/principal findings: Overexpression of Hec1 could never be obtained in HeLa clones inducibly expressing C-terminally tagged Hec1 or untagged Hec1, suggesting that Hec1 cellular levels are tightly controlled. On the contrary, a chimeric protein with an EGFP tag fused to the Hec1 N-terminus accumulated in cells and disrupted mitotic division. EGFP- Hec1 cells underwent altered chromosome segregation within multipolar spindles that originated from centriole splitting. We found that EGFP-Hec1 assembled a mutant Ndc80 complex that was unable to rescue the mitotic phenotypes of Hec1 depletion. Kinetochores harboring EGFP-Hec1 formed persisting lateral microtubule-kinetochore interactions that recruited the plus-end depolymerase MCAK and the microtubule stabilizing protein HURP on K-fibers. In these conditions the plus-end kinesin CENP-E was preferentially retained at kinetochores. RNAi-mediated CENP-E depletion further demonstrated that CENP-E function was required for multipolar spindle formation in EGFP-Hec1 expressing cells.
Conclusions/significance: Our study suggests that modifications on Hec1 N-terminal tail can alter kinetochore-microtubule attachment stability and influence Ndc80 complex function independently from the intracellular levels of the protein. N-terminally modified Hec1 promotes spindle pole fragmentation by CENP-E-mediated plus-end directed kinetochore pulling forces that disrupt the fine balance of kinetochore- and centrosome-associated forces regulating spindle bipolarity. Overall, our findings support a model in which centrosome integrity is influenced by the pathways regulating kinetochore-microtubule attachment stability.
Conflict of interest statement
Figures



References
-
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. - PubMed
-
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. - PubMed
-
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. - PubMed
-
- Cimini D, Degrassi F. Aneuploidy: a matter of bad connections. Trends Cell Biol. 2005;15:442–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

