MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction
- PMID: 21297988
- PMCID: PMC3030577
- DOI: 10.1371/journal.pone.0016411
MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction
Abstract
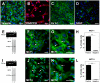
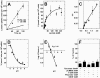
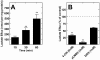
Metabolic interaction via lactate between glial cells and neurons has been proposed as one of the mechanisms involved in hypothalamic glucosensing. We have postulated that hypothalamic glial cells, also known as tanycytes, produce lactate by glycolytic metabolism of glucose. Transfer of lactate to neighboring neurons stimulates ATP synthesis and thus contributes to their activation. Because destruction of third ventricle (III-V) tanycytes is sufficient to alter blood glucose levels and food intake in rats, it is hypothesized that tanycytes are involved in the hypothalamic glucose sensing mechanism. Here, we demonstrate the presence and function of monocarboxylate transporters (MCTs) in tanycytes. Specifically, MCT1 and MCT4 expression as well as their distribution were analyzed in Sprague Dawley rat brain, and we demonstrate that both transporters are expressed in tanycytes. Using primary tanycyte cultures, kinetic analyses and sensitivity to inhibitors were undertaken to confirm that MCT1 and MCT4 were functional for lactate influx. Additionally, physiological concentrations of glucose induced lactate efflux in cultured tanycytes, which was inhibited by classical MCT inhibitors. Because the expression of both MCT1 and MCT4 has been linked to lactate efflux, we propose that tanycytes participate in glucose sensing based on a metabolic interaction with neurons of the arcuate nucleus, which are stimulated by lactate released from MCT1 and MCT4-expressing tanycytes.
Conflict of interest statement
Figures
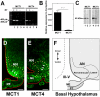
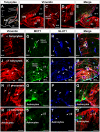
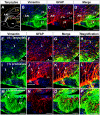
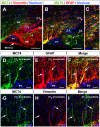
References
-
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. - PubMed
-
- Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol. 1999;276:R1223–1231. - PubMed
-
- Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. - PubMed
-
- Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2005;54:15–22. - PubMed
-
- Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, et al. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

