Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus
- PMID: 21297989
- PMCID: PMC3030580
- DOI: 10.1371/journal.pone.0016458
Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus
Abstract
Respiratory syncytial virus (RSV) causes recurrent infections throughout life. Vaccine development may depend upon understanding the molecular basis for induction of ineffective immunity. Because dendritic cells (DCs) are critically involved in early responses to infection, their interaction with RSV may determine the immunological outcome of RSV infection. Therefore, we investigated the ability of RSV to infect and activate primary mDCs and pDCs using recombinant RSV expressing green fluorescent protein (GFP). At a multiplicity of infection of 5, initial studies demonstrated ∼6.8% of mDC1 and ∼0.9% pDCs were infected. We extended these studies to include CD1c(-)CD141(+) mDC2, finding mDC2 infected at similar frequencies as mDC1. Both infected and uninfected cells upregulated phenotypic markers of maturation. Divalent cations were required for infection and maturation, but maturation did not require viral replication. There is evidence that attachment and entry/replication processes exert distinct effects on DC activation. Cell-specific patterns of RSV-induced maturation and cytokine production were detected in mDC1, mDC2, and pDC. We also demonstrate for the first time that RSV induces significant TIMP-2 production in all DC subsets. Defining the influence of RSV on the function of selected DC subsets may improve the likelihood of achieving protective vaccine-induced immunity.
Conflict of interest statement
Figures
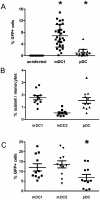
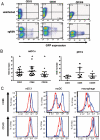

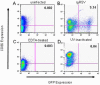
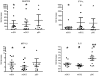
References
-
- Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. 2003;22:S76–S82. - PubMed
-
- Schauer U, Hoffjan S, Bittscheidt J, Köchling A, Hemmis S, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20:1277–1283. - PubMed
-
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Resp Crit Care Med. 2000;161:1501–1507. - PubMed
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

