Allelic diversity of the Plasmodium falciparum erythrocyte membrane protein 1 entails variant-specific red cell surface epitopes
- PMID: 21298021
- PMCID: PMC3029348
- DOI: 10.1371/journal.pone.0016544
Allelic diversity of the Plasmodium falciparum erythrocyte membrane protein 1 entails variant-specific red cell surface epitopes
Abstract
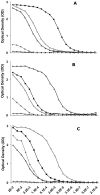
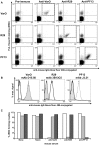
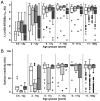
The clonally variant Plasmodium falciparum PfEMP1 adhesin is a virulence factor and a prime target of humoral immunity. It is encoded by a repertoire of functionally differentiated var genes, which display architectural diversity and allelic polymorphism. Their serological relationship is key to understanding the evolutionary constraints on this gene family and rational vaccine design. Here, we investigated the Palo Alto/VarO and IT4/R29 and 3D7/PF13_003 parasites lines. VarO and R29 form rosettes with uninfected erythrocytes, a phenotype associated with severe malaria. They express an allelic Cys2/group A NTS-DBL1α(1) PfEMP1 domain implicated in rosetting, whose 3D7 ortholog is encoded by PF13_0003. Using these three recombinant NTS-DBL1α(1) domains, we elicited antibodies in mice that were used to develop monovariant cultures by panning selection. The 3D7/PF13_0003 parasites formed rosettes, revealing a correlation between sequence identity and virulence phenotype. The antibodies cross-reacted with the allelic domains in ELISA but only minimally with the Cys4/group B/C PFL1955w NTS-DBL1α. By contrast, they were variant-specific in surface seroreactivity of the monovariant-infected red cells by FACS analysis and in rosette-disruption assays. Thus, while ELISA can differentiate serogroups, surface reactivity assays define the more restrictive serotypes. Irrespective of cumulated exposure to infection, antibodies acquired by humans living in a malaria-endemic area also displayed a variant-specific surface reactivity. Although seroprevalence exceeded 90% for each rosetting line, the kinetics of acquisition of surface-reactive antibodies differed in the younger age groups. These data indicate that humans acquire an antibody repertoire to non-overlapping serotypes within a serogroup, consistent with an antibody-driven diversification pressure at the population level. In addition, the data provide important information for vaccine design, as production of a vaccine targeting rosetting PfEMP1 adhesins will require engineering to induce variant-transcending responses or combining multiple serotypes to elicit a broad spectrum of immunity.
Conflict of interest statement
Figures





References
-
- Contamin H, Fandeur T, Rogier C, Bonnefoy S, Konate L, et al. Different genetic characteristics of Plasmodium falciparum isolates collected during successive clinical malaria episodes in Senegalese children. Am J Trop Med Hyg. 1996;54:632–643. - PubMed
-
- Marsh K, Howard RJ. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. - PubMed
-
- Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9:374–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

