The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa
- PMID: 21298031
- PMCID: PMC3029257
- DOI: 10.1371/journal.ppat.1001264
The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa
Abstract
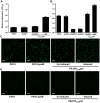
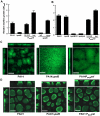
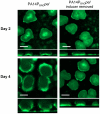
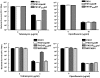
Bacterial extracellular polysaccharides are a key constituent of the extracellular matrix material of biofilms. Pseudomonas aeruginosa is a model organism for biofilm studies and produces three extracellular polysaccharides that have been implicated in biofilm development, alginate, Psl and Pel. Significant work has been conducted on the roles of alginate and Psl in biofilm development, however we know little regarding Pel. In this study, we demonstrate that Pel can serve two functions in biofilms. Using a novel assay involving optical tweezers, we demonstrate that Pel is crucial for maintaining cell-to-cell interactions in a PA14 biofilm, serving as a primary structural scaffold for the community. Deletion of pelB resulted in a severe biofilm deficiency. Interestingly, this effect is strain-specific. Loss of Pel production in the laboratory strain PAO1 resulted in no difference in attachment or biofilm development; instead Psl proved to be the primary structural polysaccharide for biofilm maturity. Furthermore, we demonstrate that Pel plays a second role by enhancing resistance to aminoglycoside antibiotics. This protection occurs only in biofilm populations. We show that expression of the pel gene cluster and PelF protein levels are enhanced during biofilm growth compared to liquid cultures. Thus, we propose that Pel is capable of playing both a structural and a protective role in P. aeruginosa biofilms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. - PubMed
-
- Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. - PubMed
-
- Drenkard E. Antimicrobial resistance of Pseudomonas aeruginosa biofilms. Microbes Infect. 2003;5:1213–1219. - PubMed
-
- Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

