Epigenetics underpinning the regulation of the CXC (ELR+) chemokines in non-small cell lung cancer
- PMID: 21298036
- PMCID: PMC3029265
- DOI: 10.1371/journal.pone.0014593
Epigenetics underpinning the regulation of the CXC (ELR+) chemokines in non-small cell lung cancer
Abstract
Background: Angiogenesis may play a role in the pathogenesis of Non-Small Cell Lung cancer (NSCLC). The CXC (ELR(+)) chemokine family are powerful promoters of the angiogenic response.
Methods: The expression of the CXC (ELR(+)) family members (CXCL1-3/GROα-γ, CXCL8/IL-8, CXCR1/2) was examined in a series of resected fresh frozen NSCLC tumours. Additionally, the expression and epigenetic regulation of these chemokines was examined in normal bronchial epithelial and NSCLC cell lines.
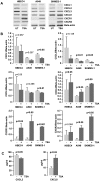
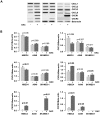
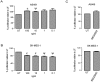
Results: Overall, expression of the chemokine ligands (CXCL1, 2, 8) and their receptors (CXCR1/2) were down regulated in tumour samples compared with normal, with the exception of CXCL3. CXCL8 and CXCR1/2 were found to be epigenetically regulated by histone post-translational modifications. Recombinant CXCL8 did not stimulate cell growth in either a normal bronchial epithelial or a squamous carcinoma cell line (SKMES-1). However, an increase was observed at 72 hours post treatment in an adenocarcinoma cell line.
Conclusions: CXC (ELR(+)) chemokines are dysregulated in NSCLC. The balance of these chemokines may be critical in the tumour microenvironment and requires further elucidation. It remains to be seen if epigenetic targeting of these pathways is a viable therapeutic option in lung cancer treatment.
Conflict of interest statement
Figures



References
-
- O'Byrne KJ, Dalgleish AG, Browning MJ, Steward WP, Harris AL. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur J Cancer. 2000;36:151–169. - PubMed
-
- Arenberg DA, Polverini PJ, Kunkel SL, Shanafelt A, Hesselgesser J, et al. The role of CXC chemokines in the regulation of angiogenesis in non-small cell lung cancer. J Leukoc Biol. 1997;62:554–562. - PubMed
-
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. - PubMed
-
- Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. - PubMed
-
- Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

