Impaired auditory-vestibular functions and behavioral abnormalities of Slitrk6-deficient mice
- PMID: 21298075
- PMCID: PMC3027700
- DOI: 10.1371/journal.pone.0016497
Impaired auditory-vestibular functions and behavioral abnormalities of Slitrk6-deficient mice
Abstract
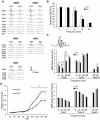
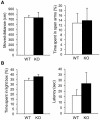
A recent study revealed that Slitrk6, a transmembrane protein containing a leucine-rich repeat domain, has a critical role in the development of the inner ear neural circuit. However, it is still unknown how the absence of Slitrk6 affects auditory and vestibular functions. In addition, the role of Slitrk6 in regions of the central nervous system, including the dorsal thalamus, has not been addressed. To understand the physiological role of Slitrk6, Slitrk6-knockout (KO) mice were subjected to systematic behavioral analyses including auditory and vestibular function tests. Compared to wild-type mice, the auditory brainstem response (ABR) of Slitrk6-KO mice indicated a mid-frequency range (8-16 kHz) hearing loss and reduction of the first ABR wave. The auditory startle response was also reduced. A vestibulo-ocular reflex (VOR) test showed decreased vertical (head movement-induced) VOR gains and normal horizontal VOR. In an open field test, locomotor activity was reduced; the tendency to be in the center region was increased, but only in the first 5 min of the test, indicating altered adaptive responses to a novel environment. Altered adaptive responses were also found in a hole-board test in which head-dip behavior was increased and advanced. Aside from these abnormalities, no clear abnormalities were noted in the mood, anxiety, learning, spatial memory, or fear memory-related behavioral tests. These results indicate that the Slitrk6-KO mouse can serve as a model of hereditary sensorineural deafness. Furthermore, the altered responses of Slitrk6-KO mice to the novel environment suggest a role of Slitrk6 in some cognitive functions.
Conflict of interest statement
Figures



References
-
- Aruga J, Mikoshiba K. Identification and characterization of Slitrk, a novel neuronal transmembrane protein family controlling neurite outgrowth. Mol Cell Neurosci. 2003;24:117–129. - PubMed
-
- Aruga J, Yokota N, Mikoshiba K. Human SLITRK family genes: genomic organization and expression profiling in normal brain and brain tumor tissue. Gene. 2003;315:87–94. - PubMed
-
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. - PubMed
-
- Aruga J. Slitrk6 expression profile in the mouse embryo and its relationship to that of Nlrr3. Gene Expr Patterns. 2003;3:727–733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

