Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study
- PMID: 21298076
- PMCID: PMC3029229
- DOI: 10.1371/journal.pcbi.1001067
Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study
Abstract
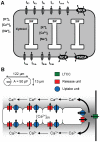
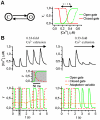
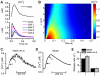
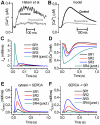
Electrophysiological studies of the human heart face the fundamental challenge that experimental data can be acquired only from patients with underlying heart disease. Regarding human atria, there exist sizable gaps in the understanding of the functional role of cellular Ca²+ dynamics, which differ crucially from that of ventricular cells, in the modulation of excitation-contraction coupling. Accordingly, the objective of this study was to develop a mathematical model of the human atrial myocyte that, in addition to the sarcolemmal (SL) ion currents, accounts for the heterogeneity of intracellular Ca²+ dynamics emerging from a structurally detailed sarcoplasmic reticulum (SR). Based on the simulation results, our model convincingly reproduces the principal characteristics of Ca²+ dynamics: 1) the biphasic increment during the upstroke of the Ca²+ transient resulting from the delay between the peripheral and central SR Ca²+ release, and 2) the relative contribution of SL Ca²+ current and SR Ca²+ release to the Ca²+ transient. In line with experimental findings, the model also replicates the strong impact of intracellular Ca²+ dynamics on the shape of the action potential. The simulation results suggest that the peripheral SR Ca²+ release sites define the interface between Ca²+ and AP, whereas the central release sites are important for the fire-diffuse-fire propagation of Ca²+ diffusion. Furthermore, our analysis predicts that the modulation of the action potential duration due to increasing heart rate is largely mediated by changes in the intracellular Na+ concentration. Finally, the results indicate that the SR Ca²+ release is a strong modulator of AP duration and, consequently, myocyte refractoriness/excitability. We conclude that the developed model is robust and reproduces many fundamental aspects of the tight coupling between SL ion currents and intracellular Ca²+ signaling. Thus, the model provides a useful framework for future studies of excitation-contraction coupling in human atrial myocytes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
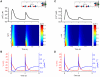
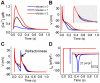
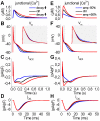

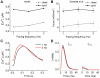
References
-
- Bers DM. Excitation-contraction coupling and cardiac contractile force. Dordrecht ; Boston: Kluwer Academic Publishers; 2001. p. xxiv, 427.
-
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol Cell Physiol. 1983;245:C1–14. - PubMed
-
- Tanaami T, Ishida H, Seguchi H, Hirota Y, Kadono T, et al. Difference in propagation of Ca2+ release in atrial and ventricular myocytes. Jpn J Physiol. 2005;55:81–91. - PubMed
-
- Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol. 2009;46:463–473. - PubMed
-
- Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92:1182–1192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

