A novel method for volumetric MRI response assessment of enhancing brain tumors
- PMID: 21298088
- PMCID: PMC3027624
- DOI: 10.1371/journal.pone.0016031
A novel method for volumetric MRI response assessment of enhancing brain tumors
Abstract
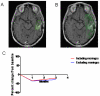
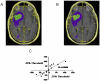
Current radiographic response criteria for brain tumors have difficulty describing changes surrounding postoperative resection cavities. Volumetric techniques may offer improved assessment, however usually are time-consuming, subjective and require expert opinion and specialized magnetic resonance imaging (MRI) sequences. We describe the application of a novel volumetric software algorithm that is nearly fully automated and uses standard T1 pre- and post-contrast MRI sequences. T1-weighted pre- and post-contrast images are automatically fused and normalized. The tumor region of interest is grossly outlined by the user. An atlas of the nasal mucosa is automatically detected and used to normalize levels of enhancement. The volume of enhancing tumor is then automatically calculated. We tested the ability of our method to calculate enhancing tumor volume with resection cavity collapse and when the enhancing tumor is obscured by subacute blood in a resection cavity. To determine variability in results, we compared narrowly-defined tumor regions with tumor regions that include adjacent meningeal enhancement and also compared different contrast enhancement threshold levels used for the automatic calculation of enhancing tumor volume. Our method quantified enhancing tumor volume despite resection cavity collapse. It detected tumor volume increase in the midst of blood products that incorrectly caused decreased measurements by other techniques. Similar trends in volume changes across scans were seen with inclusion or exclusion of meningeal enhancement and despite different automated thresholds for tissue enhancement. Our approach appears to overcome many of the challenges with response assessment of enhancing brain tumors and warrants further examination and validation.
Conflict of interest statement
Figures
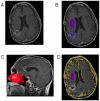
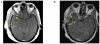
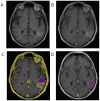
References
-
- Jaffe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245–3251. - PubMed
-
- Suzuki C, Jacobsson H, Hatschek T, Torkzad MR, Boden K, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28:329–344. - PubMed
-
- Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

