Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers
- PMID: 21298111
- PMCID: PMC3027666
- DOI: 10.1371/journal.pone.0016376
Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers
Abstract
Background: The development of insulin resistance (IR) in mouse models of obesity and type 2 diabetes mellitus (DM) is characterized by progressive accumulation of inflammatory macrophages and subpopulations of T cells in the visceral adipose. Regulatory T cells (Tregs) may play a critical role in modulating tissue inflammation via their interactions with both adaptive and innate immune mechanisms. We hypothesized that an imbalance in Tregs is a critical determinant of adipose inflammation and investigated the role of Tregs in IR/obesity through coordinated studies in mice and humans.
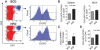
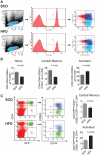
Methods and findings: Foxp3-green fluorescent protein (GFP) "knock-in" mice were randomized to a high-fat diet intervention for a duration of 12 weeks to induce DIO/IR. Morbidly obese humans without overt type 2 DM (n = 13) and lean controls (n = 7) were recruited prospectively for assessment of visceral adipose inflammation. DIO resulted in increased CD3(+)CD4(+), and CD3(+)CD8(+) cells in visceral adipose with a striking decrease in visceral adipose Tregs. Treg numbers in visceral adipose inversely correlated with CD11b(+)CD11c(+) adipose tissue macrophages (ATMs). Splenic Treg numbers were increased with up-regulation of homing receptors CXCR3 and CCR7 and marker of activation CD44. In-vitro differentiation assays showed an inhibition of Treg differentiation in response to conditioned media from inflammatory macrophages. Human visceral adipose in morbid obesity was characterized by an increase in CD11c(+) ATMs and a decrease in foxp3 expression.
Conclusions: Our experiments indicate that obesity in mice and humans results in adipose Treg depletion. These changes appear to occur via reduced local differentiation rather than impaired homing. Our findings implicate a role for Tregs as determinants of adipose inflammation.
Conflict of interest statement
Figures

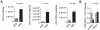


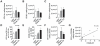
References
-
- Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. - PubMed
-
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. - PubMed
-
- Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

