Radiolabeled affibody-albumin bioconjugates for HER2-positive cancer targeting
- PMID: 21299201
- PMCID: PMC3059402
- DOI: 10.1021/bc100432h
Radiolabeled affibody-albumin bioconjugates for HER2-positive cancer targeting
Abstract
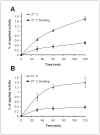
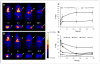
Affibody molecules have received significant attention in the fields of molecular imaging and drug development. However, Affibody scaffolds display an extremely high renal uptake, especially when modified with chelators and then labeled with radiometals. This unfavorable property may impact their use as radiotherapeutic agents in general and as imaging probes for the detection of tumors adjacent to kidneys in particular. Herein, we present a simple and generalizable strategy for reducing the renal uptake of Affibody molecules while maintaining their tumor uptake. Human serum albumin (HSA) was consecutively modified by 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono-N-hydroxysuccinimide ester (DOTA-NHS ester) and the bifunctional cross-linker sulfosuccinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (Sulfo-SMCC). The HER2 Affibody analogue, Ac-Cys-Z(HER2:342), was covalently conjugated with HSA, and the resulting bioconjugate DOTA-HSA-Z(HER2:342) was further radiolabeled with ⁶⁴Cu and ¹¹¹In and evaluated in vitro and in vivo. Radiolabeled DOTA-HSA-Z(HER2:342) conjugates displayed a significant and specific cell uptake into SKOV3 cell cultures. Positron emission tomography (PET) investigations using ⁶⁴Cu-DOTA-HSA-Z(HER2:342) were performed in SKOV3 tumor-bearing nude mice. High tumor uptake values (>14% ID/g at 24 and 48 h) and high liver accumulations but low kidney accumulations were observed. Biodistribution studies and single-photon emission computed tomography (SPECT) investigations using ¹¹¹In-DOTA-HSA-Z(HER2:342) validated these results. At 24 h post injection, the biodistribution data revealed high tumor (16.26% ID/g) and liver (14.11% ID/g) uptake but relatively low kidney uptake (6.06% ID/g). Blocking studies with coinjected, nonlabeled Ac-Cys-Z(HER2:342) confirmed the in vivo specificity of HER2. Radiolabeled DOTA-HSA-Z(HER2:342) Affibody conjugates are promising SPECT and PET-type probes for the imaging of HER2 positive cancer. More importantly, DOTA-HSA-Z(HER2:342) is suitable for labeling with therapeutic radionuclides (e.g., ⁹⁰Y or ¹⁷⁷Lu) for treatment studies. The approach of using HSA to optimize the pharmacokinetics and biodistribution profile of Affibodies may be extended to the design of many other targeting molecules.
Figures





References
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. - PubMed
-
- Schneider PM, Hung MC, Chiocca SM, Manning J, Zhao XY, Fang K, Roth JA. Differential expression of the c-erbB-2 gene in human small cell and non-small cell lung cancer. Cancer Res. 1989;49:4968–71. - PubMed
-
- Yokota J, Yamamoto T, Miyajima N, Toyoshima K, Nomura N, Sakamoto H, Yoshida T, Terada M, Sugimura T. Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene. 1988;2:283–7. - PubMed
-
- Nilsson FY, Tolmachev V. Affibody molecules: new protein domains for molecular imaging and targeted tumor therapy. Curr Opin Drug Discov Devel. 2007;10:167–75. - PubMed
-
- Nord K, Gunneriusson E, Ringdahl J, Stahl S, Uhlen M, Nygren PA. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat Biotechnol. 1997;15:772–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

