Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation
- PMID: 21300024
- PMCID: PMC3049841
- DOI: 10.1016/j.bbrc.2011.02.011
Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation
Abstract
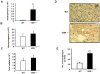
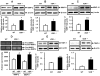
Deficiency of vitamin D is associated with accelerated decline in lung function. Vitamin D is a ligand for nuclear hormone vitamin D receptor (VDR), and upon binding it modulates various cellular functions. The level of VDR is reduced in lungs of patients with chronic obstructive pulmonary disease (COPD) which led us to hypothesize that deficiency of VDR leads to significant alterations in lung phenotype that are characteristics of COPD/emphysema associated with increased inflammatory response. We found that VDR knock-out (VDR(-/-)) mice had increased influx of inflammatory cells, phospho-acetylation of nuclear factor-kappaB (NF-κB) associated with increased proinflammatory mediators, and up-regulation of matrix metalloproteinases (MMPs) MMP-2, MMP-9, and MMP-12 in the lung. This was associated with emphysema and decline in lung function associated with lymphoid aggregates formation compared to WT mice. These findings suggest that deficiency of VDR in mouse lung can lead to an early onset of emphysema/COPD because of chronic inflammation, immune dysregulation, and lung destruction.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. - PubMed
-
- Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. - PubMed
-
- Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES001247/ES/NIEHS NIH HHS/United States
- ES-01247/ES/NIEHS NIH HHS/United States
- R03 DK089010/DK/NIDDK NIH HHS/United States
- R01 HL085613/HL/NHLBI NIH HHS/United States
- R01 HL092842/HL/NHLBI NIH HHS/United States
- R03DK089010-01/DK/NIDDK NIH HHS/United States
- DK075386-0251/DK/NIDDK NIH HHS/United States
- R01 HL097751/HL/NHLBI NIH HHS/United States
- 1R01HL097751/HL/NHLBI NIH HHS/United States
- K01 DK075386/DK/NIDDK NIH HHS/United States
- 1R01HL092842/HL/NHLBI NIH HHS/United States
- 1R01HL085613/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

