Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment
- PMID: 21300172
- PMCID: PMC3102770
- DOI: 10.1016/j.biocel.2011.01.023
Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment
Abstract
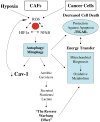
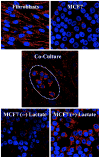
Cancer cells do not exist as pure homogeneous populations in vivo. Instead they are embedded in "cancer cell nests" that are surrounded by stromal cells, especially cancer associated fibroblasts. Thus, it is not unreasonable to suspect that stromal fibroblasts could influence the metabolism of adjacent cancer cells, and visa versa. In accordance with this idea, we have recently proposed that the Warburg effect in cancer cells may be due to culturing cancer cells by themselves, out of their normal stromal context or tumor microenvironment. In fact, when cancer cells are co-cultured with fibroblasts, then cancer cells increase their mitochondrial mass, while fibroblasts lose their mitochondria. An in depth analysis of this phenomenon reveals that aggressive cancer cells are "parasites" that use oxidative stress as a "weapon" to extract nutrients from surrounding stromal cells. Oxidative stress in fibroblasts induces the autophagic destruction of mitochondria, by mitophagy. Then, stromal cells are forced to undergo aerobic glycolysis, and produce energy-rich nutrients (such as lactate and ketones) to "feed" cancer cells. This mechanism would allow cancer cells to seed anywhere, without blood vessels as a food source, as they could simply induce oxidative stress wherever they go, explaining how cancer cells survive during metastasis. We suggest that stromal catabolism, via autophagy and mitophagy, fuels the anabolic growth of tumor cells, promoting tumor progression and metastasis. We have previously termed this new paradigm "The Autophagic Tumor Stroma Model of Cancer Metabolism", or the "Reverse Warburg Effect". We also discuss how glutamine addiction (glutaminolysis) in cancer cells fits well with this new model, by promoting oxidative mitochondrial metabolism in aggressive cancer cells.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function.Semin Oncol. 2014 Apr;41(2):195-216. doi: 10.1053/j.seminoncol.2014.03.002. Epub 2014 Mar 5. Semin Oncol. 2014. PMID: 24787293 Review.
-
Pyruvate kinase expression (PKM1 and PKM2) in cancer-associated fibroblasts drives stromal nutrient production and tumor growth.Cancer Biol Ther. 2011 Dec 15;12(12):1101-13. doi: 10.4161/cbt.12.12.18703. Epub 2011 Dec 15. Cancer Biol Ther. 2011. PMID: 22236875 Free PMC article.
-
Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment.Breast Cancer Res. 2011 Jul 8;13(4):213. doi: 10.1186/bcr2892. Breast Cancer Res. 2011. PMID: 21867571 Free PMC article. Review.
-
Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: implications for preventing chemotherapy resistance.Cancer Biol Ther. 2011 Dec 15;12(12):1085-97. doi: 10.4161/cbt.12.12.18671. Epub 2011 Dec 15. Cancer Biol Ther. 2011. PMID: 22236876 Free PMC article.
-
Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: connecting TGF-β signaling with "Warburg-like" cancer metabolism and L-lactate production.Cell Cycle. 2012 Aug 15;11(16):3019-35. doi: 10.4161/cc.21384. Epub 2012 Aug 9. Cell Cycle. 2012. PMID: 22874531 Free PMC article.
Cited by
-
Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection.Cell Cycle. 2011 Dec 1;10(23):4065-73. doi: 10.4161/cc.10.23.18254. Epub 2011 Dec 1. Cell Cycle. 2011. PMID: 22129993 Free PMC article.
-
Mitochondrial fission induces glycolytic reprogramming in cancer-associated myofibroblasts, driving stromal lactate production, and early tumor growth.Oncotarget. 2012 Aug;3(8):798-810. doi: 10.18632/oncotarget.574. Oncotarget. 2012. PMID: 22878233 Free PMC article.
-
Autophagy: a targetable linchpin of cancer cell metabolism.Trends Endocrinol Metab. 2013 Apr;24(4):209-17. doi: 10.1016/j.tem.2013.01.008. Epub 2013 Mar 6. Trends Endocrinol Metab. 2013. PMID: 23474062 Free PMC article. Review.
-
Mechanisms and Implications of Metabolic Heterogeneity in Cancer.Cell Metab. 2019 Sep 3;30(3):434-446. doi: 10.1016/j.cmet.2019.08.013. Cell Metab. 2019. PMID: 31484055 Free PMC article. Review.
-
Development of Novel Silyl Cyanocinnamic Acid Derivatives as Metabolic Plasticity Inhibitors for Cancer Treatment.Sci Rep. 2019 Dec 4;9(1):18266. doi: 10.1038/s41598-019-54709-7. Sci Rep. 2019. PMID: 31797891 Free PMC article.
References
-
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA075503/CA/NCI NIH HHS/United States
- R01 CA098779/CA/NCI NIH HHS/United States
- R01-CA-120876/CA/NCI NIH HHS/United States
- R01 CA120876/CA/NCI NIH HHS/United States
- R01-CA-70896/CA/NCI NIH HHS/United States
- R01-CA-098779/CA/NCI NIH HHS/United States
- R01-CA-86072/CA/NCI NIH HHS/United States
- R01-AR-055660/AR/NIAMS NIH HHS/United States
- R01-CA-080250/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- R01 CA107382/CA/NCI NIH HHS/United States
- P30 CA056036/CA/NCI NIH HHS/United States
- P30-CA-56036/CA/NCI NIH HHS/United States
- R01-CA-107382/CA/NCI NIH HHS/United States
- R01 AR055660/AR/NIAMS NIH HHS/United States
- R01-CA-75503/CA/NCI NIH HHS/United States
- R01 CA080250/CA/NCI NIH HHS/United States
- R01 CA086072/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

