Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome
- PMID: 21300747
- PMCID: PMC3099023
- DOI: 10.1113/jphysiol.2010.198531
Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome
Abstract
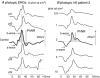
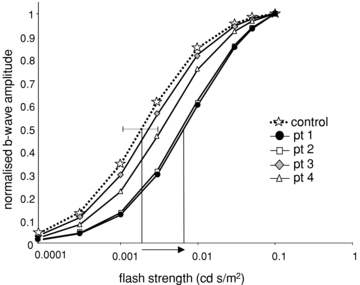
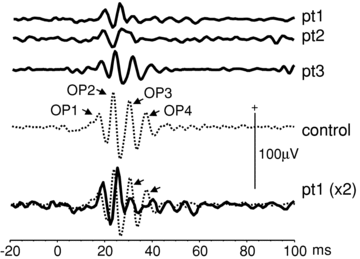
The K+ channel expressed by the KCNJ10 gene (Kir4.1) has previously demonstrated importance in retinal function in animal experiments. Recently, mutations in KCNJ10 were recognised as pathogenic in man, causing a constellation of symptoms, including epilepsy, ataxia, sensorineural deafness and a renal tubulopathy designated as EAST syndrome. We have studied the impact of KCNJ10 mutations on the human electroretinogram (ERG) in four unrelated patients with EAST syndrome. Corneal ganzfeld ERGs were elicited in response to flash stimuli of strengths of 0.001–10 phot cd s/m2 presented scotopically, and 0.3–10 phot cd s/m2 presented photopically. ERG waveforms from light-adapted retinae of all patients showed reduced amplitudes of the photopic negative response (PhNR) (P < 0.001). The photopic ERGs showed a delay in b-wave time to peak, but the photopic hill, i.e. the relative variation of time to peak and amplitude with luminance flash strength, was preserved. Scotopic ERGs to flash strengths 0.01 to 0.1 phot cd s/m2 showed a delay of up to 20 ms before the onset of the b-wave in two patients compared to controls. Stimulus–response functions were fitted by Michaelis–Menten equations and showed significantly lower retinal sensitivity in two patients than in controls (P < 0.001). Our study for the first time in the human ERG shows changes in association with KCNJ10 mutations affecting a Muller cell K+ channel. These data illustrate the role of KCNJ10 function in the physiology of proximal and possibly also the distal human retina.
Figures




References
-
- Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54:143–160. - PubMed
-
- Connors NC, Kofuji P. Potassium channel Kir4.1 macromolecular complex in retinal glial cells. Glia. 2006;53:124–131. - PubMed
-
- Dong CJ, Agey P, Hare WA. Origins of the electroretinogram oscillatory potentials in the rabbit retina. Vis Neurosci. 2004;21:533–543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

