Is connexin36 critical for GABAergic hypersynchronization in the hippocampus?
- PMID: 21300748
- PMCID: PMC3099022
- DOI: 10.1113/jphysiol.2010.201491
Is connexin36 critical for GABAergic hypersynchronization in the hippocampus?
Abstract
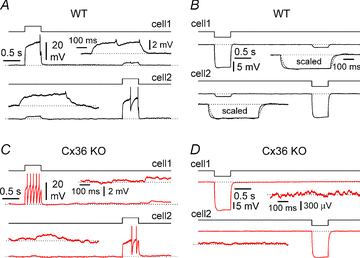
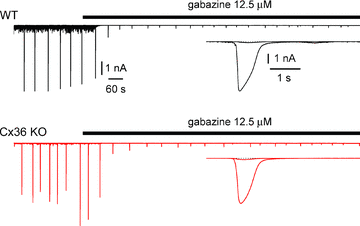
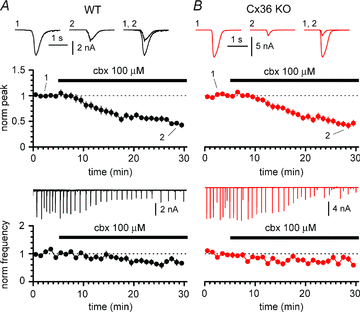
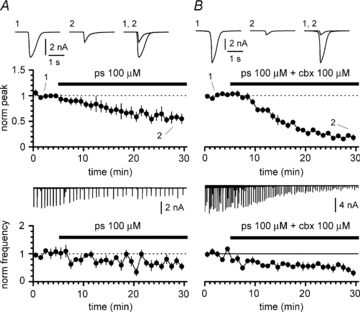
Synchronous bursting of cortical GABAergic interneurons is important in epilepsies associated with excitatory GABAergic signalling. If electrical coupling was critical for the generation of this pathological activity, then the development of selective blockers of connexin36-based interneuronal gap junctions could be of therapeutic value. We have addressed this issue in the 4-aminopyridine model of epilepsy in vitro by comparing GABAergic epileptiform currents and their sensitivity to gap junction blockers in wild-type vs. connexin36 knockout mice. Although electrical coupling was abolished in stratum lacunosum-moleculare interneurons from knockout animals, epileptiform currents were not eliminated. Furthermore, epileptiform currents propagated similarly across hippocampal layers in the two genotypic groups. Blockade of electrical coupling with carbenoxolone suppressed amplitude, frequency and half-width of the epileptiform currents both in wild-type and in knockout animals, whereas mefloquine had no effects. Carbenoxolone also depressed responses to exogenous and synaptic GABA application onto interneurons. We conclude that, in the 4-aminopyridine model of epilepsy in vitro, connexin36 is not critical for the generation of epileptiform discharges in GABAergic networks and that the observed antiepileptic effects of carbenoxolone are likely to be due to blockade of GABAA receptors and not of connexin36-based gap junctions. Lastly, because of its chemical structure and its effects on amplitude and kinetics of GABAergic currents, we tested the hypothesis that carbenoxolone acted via specific sites on GABAA receptors, such as the one mediating the effects of the neurosteroid pregnenolone sulfate, or the allosteric regulatory site of benzodiazepines/β-carbolines. Our results suggest that neither of these is involved.
Figures










References
-
- Avoli M. GABA-mediated synchronous potentials and seizure generation. Epilepsia. 1996;37:1035–1042. - PubMed
-
- Avoli M, D'Antuono, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. - PubMed
-
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. - PubMed
-
- Bennett MVL. Electrical transmission: a functional analysis and comparison to chemical transmission. In: Kandel EK, editor. Handbook of Physiology, section 1, The Nervous System, vol. I, Cellular Biology of Neurons. Baltimore: Williams & Wilkins; 1977. pp. 357–416.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

