Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors
- PMID: 21300762
- PMCID: PMC5244484
- DOI: 10.1158/1078-0432.CCR-10-2745
Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors
Abstract
Purpose: The hedgehog (Hh) signaling pathway, a key regulator of cell growth and differentiation during development is implicated in pathogenesis of certain cancers. Vismodegib (GDC-0449) is a small-molecule inhibitor of smoothened, a key component of Hh signaling. This phase I trial assessed GDC-0449 treatment in patients with solid tumors refractory to current therapies or for which no standard therapy existed.
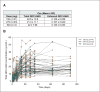
Experimental design: Sixty-eight patients received GDC-0449 at 150 mg/d (n = 41), 270 mg/d (n = 23), or 540 mg/d (n = 4). Adverse events, tumor responses, pharmacokinetics, and pharmacodynamic down-modulation of GLI1 expression in noninvolved skin were assessed.
Results: Thirty-three of 68 patients had advanced basal cell carcinoma (BCC), 8 had pancreatic cancer, 1 had medulloblastoma; 17 other types of cancer were also represented. GDC-0449 was generally well-tolerated. Six patients (8.8%) experienced 7 grade 4 events (hyponatremia, fatigue, pyelonephritis, presyncope, resectable pancreatic adenocarcinoma, and paranoia with hyperglycemia), and 27.9% of patients experienced a grade 3 event [most commonly hyponatremia (10.3%), abdominal pain (7.4%), and fatigue (5.9%)]. No maximum tolerated dose was reached. The recommended phase II dose was 150 mg/d, based on achievement of maximal plasma concentration and pharmacodynamic response at this dose. Tumor responses were observed in 20 patients (19 with BCC and 1 unconfirmed response in medulloblastoma), 14 patients had stable disease as best response, and 28 had progressive disease. Evidence of GLI1 down-modulation was observed in noninvolved skin.
Conclusions: GDC-0449 has an acceptable safety profile and encouraging anti-tumor activity in advanced BCC and medulloblastoma. Further study in these and other cancer types is warranted.
©2011 AACR.
Conflict of interest statement
of Potential Conflicts of Interest The other authors report no conflicts of interest.
Figures


Comment in
-
Targeting the Hedgehog pathway in cancer: can the spines be smoothened?Clin Cancer Res. 2011 Apr 15;17(8):2071-3. doi: 10.1158/1078-0432.CCR-11-0211. Epub 2011 Mar 2. Clin Cancer Res. 2011. PMID: 21367749
References
-
- Johnson RL, Rothman AL, XIe J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–71. - PubMed
-
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. - PubMed
-
- Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–45. - PubMed
-
- Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

