Preterm infants have deficient monocyte and lymphocyte cytokine responses to group B streptococcus
- PMID: 21300777
- PMCID: PMC3067554
- DOI: 10.1128/IAI.00535-10
Preterm infants have deficient monocyte and lymphocyte cytokine responses to group B streptococcus
Abstract
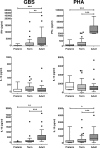
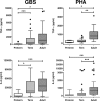
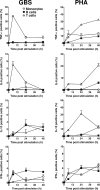
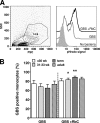
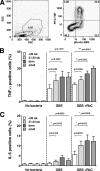
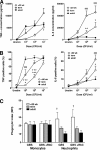
Group B streptococcus (GBS) is an important cause of early- and late-onset sepsis in the newborn. Preterm infants have markedly increased susceptibility and worse outcomes, but their immunological responses to GBS are poorly defined. We compared mononuclear cell and whole-blood cytokine responses to heat-killed GBS (HKGBS) of preterm infants (gestational age [GA], 26 to 33 weeks), term infants, and healthy adults. We investigated the kinetics and cell source of induced cytokines and quantified HKGBS phagocytosis. HKGBS-induced tumor necrosis factor (TNF) and interleukin 6 (IL-6) secretion was significantly impaired in preterm infants compared to that in term infants and adults. These cytokines were predominantly monocytic in origin, and production was intrinsically linked to HKGBS phagocytosis. Very preterm infants (GA, <30 weeks) had fewer cytokine-producing monocytes, but nonopsonic phagocytosis ability was comparable to that for term infants and adults. Exogenous complement supplementation increased phagocytosis in all groups, as well as the proportion of preterm monocytes producing IL-6, but for very preterm infants, responses were still deficient. Similar defective preterm monocyte responses were observed in fresh whole cord blood stimulated with live GBS. Lymphocyte-associated cytokines were significantly deficient for both preterm and term infants compared to levels for adults. These findings indicate that a subset of preterm monocytes do not respond to GBS, a defect compounded by generalized weaker lymphocyte responses in newborns. Together these deficient responses may increase the susceptibility of preterm infants to GBS infection.
Figures
References
-
- Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today 20:330-335. - PubMed
-
- Adkins, B. 2000. Development of neonatal Th1/Th2 function. Int. Rev. Immunol. 19:157-171. - PubMed
-
- Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. - PubMed
-
- Bektas, S., B. Goetze, and C. P. Speer. 1990. Decreased adherence, chemotaxis and phagocytic activities of neutrophils from preterm neonates. Acta Paediatr. Scand. 79:1031-1038. - PubMed
-
- Bialek, R., and P. Bartmann. 1998. Is there an effect of immunoglobulins and G-CSF on neutrophil phagocytic activity in preterm infants? Infection 26:375-378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

