Investigation of the functional link between ATM and NBS1 in the DNA damage response in the mouse cerebellum
- PMID: 21300797
- PMCID: PMC3083204
- DOI: 10.1074/jbc.M110.204172
Investigation of the functional link between ATM and NBS1 in the DNA damage response in the mouse cerebellum
Abstract
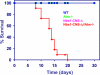
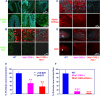
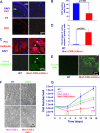
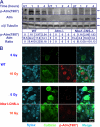
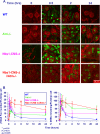
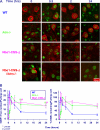
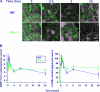
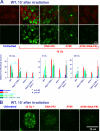
Ataxia-telangiectasia (A-T) and Nijmegen breakage syndrome (NBS) are related genomic instability syndromes characterized by neurological deficits. The NBS1 protein that is defective in NBS is a component of the Mre11/RAD50/NBS1 (MRN) complex, which plays a major role in the early phase of the complex cellular response to double strand breaks (DSBs) in the DNA. Among others, Mre11/RAD50/NBS1 is required for timely activation of the protein kinase ATM (A-T, mutated), which is missing or inactivated in patients with A-T. Understanding the molecular pathology of A-T, primarily its cardinal symptom, cerebellar degeneration, requires investigation of the DSB response in cerebellar neurons, particularly Purkinje cells, which are the first to be lost in A-T patients. Cerebellar cultures derived from mice with different mutations in DNA damage response genes is a useful experimental system to study malfunctioning of the damage response in the nervous system. To clarify the interrelations between murine Nbs1 and Atm, we generated a mouse strain with specific disruption of the Nbs1 gene in the central nervous system on the background of general Atm deficiency (Nbs1-CNS-Δ//Atm(-/-)). This genotype exacerbated several features of both conditions and led to a markedly reduced life span, dramatic decline in the number of cerebellar granule neurons with considerable cerebellar disorganization, abolishment of the white matter, severe reduction in glial cell proliferation, and delayed DSB repair in cerebellar tissue. Combined loss of Nbs1 and Atm in the CNS significantly abrogated the DSB response compared with the single mutation genotypes. Importantly, the data indicate that Atm has cellular roles not regulated by Nbs1 in the murine cerebellum.
Figures

Similar articles
-
Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1219-25. doi: 10.1016/j.dnarep.2004.04.009. DNA Repair (Amst). 2004. PMID: 15279810 Review.
-
Defective Mre11-dependent activation of Chk2 by ataxia telangiectasia mutated in colorectal carcinoma cells in response to replication-dependent DNA double strand breaks.J Biol Chem. 2006 Oct 13;281(41):30814-23. doi: 10.1074/jbc.M603747200. Epub 2006 Aug 10. J Biol Chem. 2006. PMID: 16905549
-
ATM and the Mre11-Rad50-Nbs1 complex respond to nucleoside analogue-induced stalled replication forks and contribute to drug resistance.Cancer Res. 2008 Oct 1;68(19):7947-55. doi: 10.1158/0008-5472.CAN-08-0971. Cancer Res. 2008. PMID: 18829552 Free PMC article.
-
Requirement of the MRN complex for ATM activation by DNA damage.EMBO J. 2003 Oct 15;22(20):5612-21. doi: 10.1093/emboj/cdg541. EMBO J. 2003. PMID: 14532133 Free PMC article.
-
Mystery of DNA repair: the role of the MRN complex and ATM kinase in DNA damage repair.J Appl Genet. 2008;49(4):383-96. doi: 10.1007/BF03195638. J Appl Genet. 2008. PMID: 19029686 Review.
Cited by
-
ATM in breast and brain tumors: a comprehensive review.Cancer Biol Med. 2018 Aug;15(3):210-227. doi: 10.20892/j.issn.2095-3941.2018.0022. Cancer Biol Med. 2018. PMID: 30197789 Free PMC article.
-
DNA damage in the oligodendrocyte lineage and its role in brain aging.Mech Ageing Dev. 2017 Jan;161(Pt A):37-50. doi: 10.1016/j.mad.2016.05.006. Epub 2016 May 26. Mech Ageing Dev. 2017. PMID: 27235538 Free PMC article. Review.
-
The ATM signaling network in development and disease.Front Genet. 2013 Mar 25;4:37. doi: 10.3389/fgene.2013.00037. eCollection 2013. Front Genet. 2013. PMID: 23532176 Free PMC article.
-
Inactive Atm abrogates DSB repair in mouse cerebellum more than does Atm loss, without causing a neurological phenotype.DNA Repair (Amst). 2018 Dec;72:10-17. doi: 10.1016/j.dnarep.2018.10.001. Epub 2018 Oct 11. DNA Repair (Amst). 2018. PMID: 30348496 Free PMC article.
-
Purkinje cell degeneration in pcd mice reveals large scale chromatin reorganization and gene silencing linked to defective DNA repair.J Biol Chem. 2011 Aug 12;286(32):28287-302. doi: 10.1074/jbc.M111.246041. Epub 2011 Jun 23. J Biol Chem. 2011. PMID: 21700704 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

