Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia
- PMID: 21300830
- PMCID: PMC3067164
- DOI: 10.1128/AAC.01330-10
Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia
Abstract
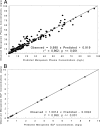
Antibiotic penetration to the infection site is critical for obtaining a good clinical outcome in patients with ventilator-associated pneumonia (VAP). Surprisingly few studies have quantified the penetration of β-lactam agents into the lung, as measured by the ratio of area under the concentration-time curve (AUC) in epithelial lining fluid (ELF) to AUC in plasma (AUC(ELF)/AUC(plasma) ratio). These have typically involved noninfected patients. This study examines the penetration and pharmacodynamics of meropenem in the ELF among patients with VAP. Meropenem plasma and ELF concentration-time data were obtained from patients in a multicenter clinical trial. Concentration-time profiles in plasma and ELF were simultaneously modeled using a three-compartment model with zero-order infusion and first-order elimination and transfer (big nonparametric adaptive grid [BigNPAG]). A Monte Carlo simulation was performed to estimate the range of ELF/plasma penetration ratios one would expect to observe in patients with VAP, as measured by the AUC(ELF)/AUC(plasma) ratio. The range of AUC(ELF)/AUC(plasma) penetration ratios predicted by the Monte Carlo simulation was large. The 10th percentile of lung penetration was 3.7%, while the 90th percentile of penetration was 178%. The variability of ELF penetration is such that if relatively high ELF exposure targets are required to attain multilog kill or resistance suppression for bacteria like Pseudomonas aeruginosa, then even receiving the largest licensed dose of meropenem with an optimal prolonged infusion may not result in target attainment for a substantial fraction of the population.
Figures

Comment in
-
Determination of meropenem penetration into the lung from Sparse data.Antimicrob Agents Chemother. 2011 Dec;55(12):5959-61. doi: 10.1128/AAC.05066-11. Antimicrob Agents Chemother. 2011. PMID: 22081725 Free PMC article. No abstract available.
References
-
- American Thoracic Society. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. - PubMed
-
- Baldwin, D. R., R. Wise, J. M. Andrews, and D. Honeybourne. 1992. Concentrations of cefpodoxime in serum and bronchial mucosal biopsies. J. Antimicrob. Chemother. 30:67-71. - PubMed
-
- Boselli, E., et al. 2003. Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit. Care Med. 31:2102-2106. - PubMed
-
- Boselli, E., et al. 2008. Alveolar concentrations of piperacillin/tazobactam administered in continuous infusion to patients with ventilator-associated pneumonia. Crit. Care Med. 36:1500-1506. - PubMed
-
- Boselli, E., et al. 2004. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 30:989-991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

