Skint-1 is a highly specific, unique selecting component for epidermal T cells
- PMID: 21300860
- PMCID: PMC3044407
- DOI: 10.1073/pnas.1010890108
Skint-1 is a highly specific, unique selecting component for epidermal T cells
Abstract
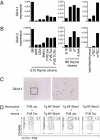
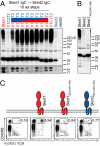
αβ T-cell repertoire selection is mediated by peptide-MHC complexes presented by thymic epithelial or myeloid cells, and by lipid-CD1 complexes expressed by thymocytes. γδ T-cell repertoire selection, by contrast, is largely unresolved. Mice mutant for Skint-1, a unique Ig superfamily gene, do not develop canonical Vγ5Vδ1(+) dendritic epidermal T cells. This study shows that transgenic Skint-1, across a broad range of expression levels, precisely and selectively determines the Vγ5Vδ1(+) dendritic epidermal T-cell compartment. Skint-1 is expressed by medullary thymic epithelial cells, and unlike lipid-CD1 complexes, must be expressed by stromal cells to function efficiently. Its unusual transmembrane-cytoplasmic regions severely limit cell surface expression, yet increasing this or, conversely, retaining Skint1 intracellularly markedly compromises function. Each Skint1 domain appears nonredundant, including a unique decamer specifying IgV-domain processing. This investigation of Skint-1 biology points to complex events underpinning the positive selection of an intraepithelial γδ repertoire.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hayday AC, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: Exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. - PubMed
-
- Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335:443–445. - PubMed
-
- Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat Rev Immunol. 2003;3:233–242. - PubMed
-
- Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

