Metabolic cross-talk allows labeling of O-linked beta-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway
- PMID: 21300897
- PMCID: PMC3044403
- DOI: 10.1073/pnas.1010045108
Metabolic cross-talk allows labeling of O-linked beta-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway
Abstract
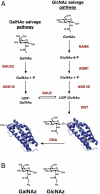
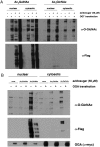
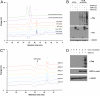
Hundreds of mammalian nuclear and cytoplasmic proteins are reversibly glycosylated by O-linked β-N-acetylglucosamine (O-GlcNAc) to regulate their function, localization, and stability. Despite its broad functional significance, the dynamic and posttranslational nature of O-GlcNAc signaling makes it challenging to study using traditional molecular and cell biological techniques alone. Here, we report that metabolic cross-talk between the N-acetylgalactosamine salvage and O-GlcNAcylation pathways can be exploited for the tagging and identification of O-GlcNAcylated proteins. We found that N-azidoacetylgalactosamine (GalNAz) is converted by endogenous mammalian biosynthetic enzymes to UDP-GalNAz and then epimerized to UDP-N-azidoacetylglucosamine (GlcNAz). O-GlcNAc transferase accepts UDP-GlcNAz as a nucleotide-sugar donor, appending an azidosugar onto its native substrates, which can then be detected by covalent labeling using azide-reactive chemical probes. In a proof-of-principle proteomics experiment, we used metabolic GalNAz labeling of human cells and a bioorthogonal chemical probe to affinity-purify and identify numerous O-GlcNAcylated proteins. Our work provides a blueprint for a wide variety of future chemical approaches to identify, visualize, and characterize dynamic O-GlcNAc signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
-
- Yuzwa SA, et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 2008;4:483–490. - PubMed
-
- Kneass ZT, Marchase RB. Neutrophils exhibit rapid agonist-induced increases in protein-associated O-GlcNAc. J Biol Chem. 2004;279:45759–45765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

