Low-resolution structure of a vesicle disrupting α-synuclein oligomer that accumulates during fibrillation
- PMID: 21300904
- PMCID: PMC3044375
- DOI: 10.1073/pnas.1013225108
Low-resolution structure of a vesicle disrupting α-synuclein oligomer that accumulates during fibrillation
Abstract
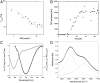
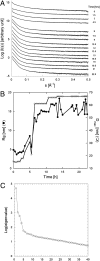
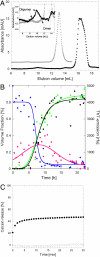
One of the major hallmarks of Parkinson disease is aggregation of the protein α-synuclein (αSN). Aggregate cytotoxicity has been linked to an oligomeric species formed at early stages in the aggregation process. Here we follow the fibrillation process of αSN in solution over time using small angle X-ray scattering and resolve four major coexisting species in the fibrillation process, namely monomer, dimer, fibril and an oligomer. By ab initio modeling to fit the data, we obtain a low-resolution structure of a symmetrical and slender αSN fibril in solution, consisting of a repeating unit with a maximal distance of 900 Å and a diameter of ∼180 Å. The same approach shows the oligomer to be shaped like a wreath, with a central channel and with dimensions corresponding to the width of the fibril. The structure, accumulation and decay of this oligomer is consistent with an on-pathway role for the oligomer in the fibrillation process. We propose an oligomer-driven αSN fibril formation mechanism, where the fibril is built from the oligomers. The wreath-shaped structure of the oligomer highlights its potential cytotoxicity by simple membrane permeabilization. This is confirmed by the ability of the purified oligomer to disrupt liposomes. Our results provide the first structural description in solution of a potentially cytotoxic oligomer, which accumulates during the fibrillation of αSN.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fink AL. The aggregation and fibrillation of alpha-synuclein. Acc Chem Res. 2006;39:628–634. - PubMed
-
- Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. - PubMed
-
- Volles MJ, et al. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–7819. - PubMed
-
- Lashuel HA, et al. Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

