Elongation factor 4 (EF4/LepA) accelerates protein synthesis at increased Mg2+ concentrations
- PMID: 21300907
- PMCID: PMC3044372
- DOI: 10.1073/pnas.1012994108
Elongation factor 4 (EF4/LepA) accelerates protein synthesis at increased Mg2+ concentrations
Abstract
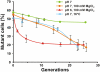
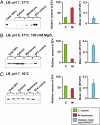
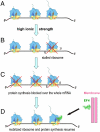
Elongation factor 4 (EF4) is one of the most conserved proteins present in bacteria as well as in mitochondria and chloroplasts of eukaryotes. Although EF4 has the unique ability to catalyze the back-translocation reaction on posttranslocation state ribosomes, the physiological role of EF4 remains unclear. Here we demonstrate that EF4 is stored at the membrane of Escherichia coli cells and released into the cytoplasm upon conditions of high ionic strength or low temperature. Under such conditions, wild-type E. coli cells overgrow mutant cells lacking the EF4 gene within 5-10 generations. Elevated intracellular Mg(2+) concentrations or low temperature retard bacterial growth and inhibit protein synthesis, probably because of formation of aberrant elongating ribosomal states. We suggest that EF4 binds to these stuck ribosomes and remobilizes them, consistent with the EF4-dependent enhancement (fivefold) in protein synthesis observed under these unfavorable conditions. The strong selective advantage conferred by the presence of EF4 at high intracellular ionic strength or low temperatures explains the ubiquitous distribution and high conservation of EF4.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nierhaus KH. The elongation cycle. In: Nierhaus KH, Wilson DN, editors. Protein Synthesis and Ribosome Structure. Weinheim, Germany: Wiley-VCH; 2004. pp. 323–366.
-
- Qin Y, et al. The highly conserved LepA is a ribosomal elongation factor that back-translocates the ribosome. Cell. 2006;127:721–733. - PubMed
-
- Chen J, et al. Legionella effectors that promote nonlytic release from protozoa. Science. 2004;303:1358–1361. - PubMed
-
- March PE, Inouye M. Characterization of the Lep operon of Escherichia coli. Identification of the promoter and the gene upstream of the signal peptidase I gene. J Biol Chem. 1985;260:7206–7213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

